On the origin of low-density neutrophils
- PMID: 32170882
- PMCID: PMC7318192
- DOI: 10.1002/JLB.5HR0120-459R
On the origin of low-density neutrophils
Abstract
Here we elaborate on the origin of low(er)-density neutrophils (LDNs) to better understand the variation found in literature. Supplemented with original data, we test the hypothesis that buoyant density of neutrophils is characterized by a spectrum that as a whole shifts to a lower density after activation. Both the 20% highest density (HDNs) and 20% lowest density (LDNs) neutrophils from healthy donors were isolated by Percoll of different densities. Using this method we found that LDNs were significantly better in T-cell suppression and bacterial containment than their 20% highest density counterparts. We found no statistically relevant differences in neutrophil survival or bacterial phagocytosis. Stimulation of healthy donor neutrophils with N-formyl-methionyl-leucyl-phenylalanine induced LDNs co-segregating with peripheral blood mononuclear cells after Ficoll separation. These in vitro induced LDNs showed increased activation markers compared to HDNs and were comparable to the activation markers found on the LDN fraction seen in patients with chronic inflammatory conditions such as present in cancer patients. This all fits with the hypothesis that the density of neutrophils is distributed in a spectrum partially coupled to maturation. Additionally a shift in this spectrum can be induced by in vitro stimulation or by activation in disease.
Keywords: LDG; granulocytes; neutrophil subsets.
© 2020 The Authors. Journal of Leukocyte Biology published by Wiley Periodicals, Inc. on behalf of Society for Leukocyte Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
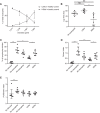
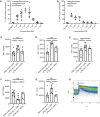

Comment in
-
Immature neutrophils in cord blood exert increased expression of genes associated with antimicrobial function.Front Immunol. 2024 Mar 26;15:1368624. doi: 10.3389/fimmu.2024.1368624. eCollection 2024. Front Immunol. 2024. PMID: 38596677 Free PMC article.
Similar articles
-
Neutrophil extracellular traps contribute to liver damage and increase defective low-density neutrophils in alcohol-associated hepatitis.J Hepatol. 2023 Jan;78(1):28-44. doi: 10.1016/j.jhep.2022.08.029. Epub 2022 Sep 5. J Hepatol. 2023. PMID: 36063965
-
Neutrophil subpopulations and their activation potential in patients with antiphospholipid syndrome and healthy individuals.Rheumatology (Oxford). 2021 Apr 6;60(4):1687-1699. doi: 10.1093/rheumatology/keaa532. Rheumatology (Oxford). 2021. PMID: 33026085 Free PMC article.
-
High Purity Isolation of Low Density Neutrophils Casts Doubt on Their Exceptionality in Health and Disease.Front Immunol. 2021 Jun 8;12:625922. doi: 10.3389/fimmu.2021.625922. eCollection 2021. Front Immunol. 2021. PMID: 34168640 Free PMC article.
-
Low-density granulocytes: a distinct class of neutrophils in systemic autoimmunity.Semin Immunopathol. 2013 Jul;35(4):455-63. doi: 10.1007/s00281-013-0375-7. Epub 2013 Apr 4. Semin Immunopathol. 2013. PMID: 23553215 Free PMC article. Review.
-
Understanding the Multifaceted Role of Neutrophils in Cancer and Autoimmune Diseases.Front Immunol. 2018 Nov 9;9:2456. doi: 10.3389/fimmu.2018.02456. eCollection 2018. Front Immunol. 2018. PMID: 30473691 Free PMC article. Review.
Cited by
-
Impact of SARS-CoV-2 infection on the recovery of peripheral blood mononuclear cells by density gradient.Sci Rep. 2021 Mar 1;11(1):4904. doi: 10.1038/s41598-021-83950-2. Sci Rep. 2021. PMID: 33649400 Free PMC article.
-
Shirebi granules ameliorate acute gouty arthritis by inhibiting NETs-induced imbalance between immunity and inflammation.Chin Med. 2024 Aug 9;19(1):105. doi: 10.1186/s13020-024-00962-6. Chin Med. 2024. PMID: 39123236 Free PMC article.
-
Single-Cell Profiling of Tumor-Associated Neutrophils in Advanced Non-Small Cell Lung Cancer.Lung Cancer (Auckl). 2023 Nov 21;14:85-99. doi: 10.2147/LCTT.S430967. eCollection 2023. Lung Cancer (Auckl). 2023. PMID: 38025400 Free PMC article.
-
Circulating Low Density Neutrophils Are Associated with Resistance to First Line Anti-PD1/PDL1 Immunotherapy in Non-Small Cell Lung Cancer.Cancers (Basel). 2022 Aug 9;14(16):3846. doi: 10.3390/cancers14163846. Cancers (Basel). 2022. PMID: 36010840 Free PMC article.
-
Neutrophils at the crossroads of acute viral infections and severity.Mol Aspects Med. 2021 Oct;81:100996. doi: 10.1016/j.mam.2021.100996. Epub 2021 Jul 18. Mol Aspects Med. 2021. PMID: 34284874 Free PMC article. Review.
References
-
- Ng LG, Ostuni R, Hidalgo A. Heterogeneity of neutrophils. Nat Rev Immunol. 2019;19:255‐265. - PubMed
-
- Hacbarth E, Kajdacsy‐Balla A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheum. 1986;29:1334‐1342. - PubMed
-
- Scapini P, Marini O, Tecchio C, et al. Human neutrophils in the saga of cellular heterogeneity: insights and open questions. Immunol Rev. 2016;273:48‐60. - PubMed
-
- Pember SO, Barnes KC, Brandt SJ, et al. Density heterogeneity of neutrophilic polymorphonuclear leukocytes: gradient fractionation and relationship to chemotactic stimulation. Blood. 1983;61:1105‐1115. - PubMed
-
- Pember SO, Kinkade JM. Differences in myeloperoxidase activity from neutrophilic polymorphonuclear leukocytes of differing density: relationship to selective exocytosis of distinct forms of the enzyme. Blood. 1983;61:1116‐1124. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

