Novel Molecular Mechanisms of Gangliosides in the Nervous System Elucidated by Genetic Engineering
- PMID: 32168753
- PMCID: PMC7139306
- DOI: 10.3390/ijms21061906
Novel Molecular Mechanisms of Gangliosides in the Nervous System Elucidated by Genetic Engineering
Abstract
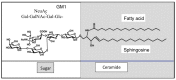
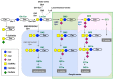
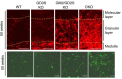
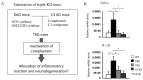
Acidic glycosphingolipids, i.e., gangliosides, are predominantly and consistently expressed in nervous tissues of vertebrates at high levels. Therefore, they are considered to be involved in the development and function of nervous systems. Recent studies involving genetic engineering of glycosyltransferase genes have revealed novel aspects of the roles of gangliosides in the regulation of nervous tissues. In this review, novel findings regarding ganglioside functions and their modes of action elucidated mainly by studies of gene knockout mice are summarized. In particular, the roles of gangliosides in the regulation of lipid rafts to maintain the integrity of nervous systems are reported with a focus on the roles in the regulation of neuro-inflammation and neurodegeneration via complement systems. In addition, recent advances in studies of congenital neurological disorders due to genetic mutations of ganglioside synthase genes and also in the techniques for the analysis of ganglioside functions are introduced.
Keywords: ganglioside; glycosphingolipid; inflammation; knockout; microdomain; neurodegeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Regulatory mechanisms of nervous systems with glycosphingolipids.Neurochem Res. 2011 Sep;36(9):1578-86. doi: 10.1007/s11064-011-0494-2. Epub 2011 May 12. Neurochem Res. 2011. PMID: 21562747 Review.
-
Gangliosides in Inflammation and Neurodegeneration.Prog Mol Biol Transl Sci. 2018;156:265-287. doi: 10.1016/bs.pmbts.2018.01.009. Epub 2018 Apr 10. Prog Mol Biol Transl Sci. 2018. PMID: 29747817 Review.
-
Gangliosides are essential in the protection of inflammation and neurodegeneration via maintenance of lipid rafts: elucidation by a series of ganglioside-deficient mutant mice.J Neurochem. 2011 Mar;116(5):926-35. doi: 10.1111/j.1471-4159.2010.07067.x. Epub 2011 Jan 12. J Neurochem. 2011. PMID: 21214571
-
Ganglioside deficiency causes inflammation and neurodegeneration via the activation of complement system in the spinal cord.J Neuroinflammation. 2014 Mar 28;11:61. doi: 10.1186/1742-2094-11-61. J Neuroinflammation. 2014. PMID: 24673754 Free PMC article.
-
Gangliosides play pivotal roles in the regulation of complement systems and in the maintenance of integrity in nerve tissues.Proc Natl Acad Sci U S A. 2009 Dec 29;106(52):22405-10. doi: 10.1073/pnas.0912336106. Epub 2009 Dec 11. Proc Natl Acad Sci U S A. 2009. PMID: 20018737 Free PMC article.
Cited by
-
The Regulatory Roles of Cerebellar Glycosphingolipid Microdomains/Lipid Rafts.Int J Mol Sci. 2023 Mar 14;24(6):5566. doi: 10.3390/ijms24065566. Int J Mol Sci. 2023. PMID: 36982638 Free PMC article. Review.
-
Editorial for Special Issue "Gangliosides: Modes of Action and Cell Fates".Int J Mol Sci. 2020 Sep 8;21(18):6552. doi: 10.3390/ijms21186552. Int J Mol Sci. 2020. PMID: 32911611 Free PMC article.
-
COVID-19-Associated Guillain-Barre Syndrome: Atypical Para-infectious Profile, Symptom Overlap, and Increased Risk of Severe Neurological Complications.SN Compr Clin Med. 2020;2(12):2702-2714. doi: 10.1007/s42399-020-00646-w. Epub 2020 Nov 21. SN Compr Clin Med. 2020. PMID: 33251483 Free PMC article. Review.
-
Lipid rafts and human diseases: why we need to target gangliosides.FEBS Open Bio. 2023 Sep;13(9):1636-1650. doi: 10.1002/2211-5463.13612. Epub 2023 Apr 20. FEBS Open Bio. 2023. PMID: 37052878 Free PMC article. Review.
-
Relation between Guillain-Barré syndrome and Covid-19: Case-Series.J Med Life. 2023 Sep;16(9):1433-1435. doi: 10.25122/jml-2023-0275. J Med Life. 2023. PMID: 38107719 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

