FANCD2 is required for the repression of germline transposable elements
- PMID: 32163912
- PMCID: PMC7193181
- DOI: 10.1530/REP-19-0436
FANCD2 is required for the repression of germline transposable elements
Abstract
The Fanconi anemia (FA) DNA damage response (DDR) pathway regulate important cellular processes such as DNA replication, cell cycle control and DNA damage repair. Here we show that FANCD2, a key member of the FA DDR pathway, interacts with several important components of the germ-cell-specific Prmt5/piRNA pathways that orchestrate the repression of transposable elements (TEs). By using the Pou5f1-eGFP reporter mice, which marks pure populations of primordial germ cells (PGCs), we demonstrate that FA deficiency results in de-repression of TEs, depletion of PGCs, and defective spermatogenesis and oogenesis. Fancd2–KO PGCs exhibited excessive DNA damage and exacerbated apoptosis. Mechanistically, we observed a significant reduction of PRMT5-catalyzed H2A/H4R3me2s marks on the LINE1 TEs in E10.5 PGCs of Fancd2-KO; Pou5f1-eGFP and Fanca-KO;Pou5f1-eGFP embryos. Furthermore, we utilized the Fancd2-KI model to show that Fancd2 and Prmt5 co-occupied the promoter of LINE1 in WT PGCs, and that this co-occupancy was lost in FA-deficient (Fanca-KO) PGCs. These results suggest that the FA pathway takes part in TE repression in early PGCs, likely through a mechanism involving Fancd2-facilitated, Prmt5-catalyzed repressive H2A/H4R3me2s marks on TEs.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures
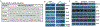

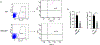

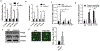
Similar articles
-
FANCB is essential in the male germline and regulates H3K9 methylation on the sex chromosomes during meiosis.Hum Mol Genet. 2015 Sep 15;24(18):5234-49. doi: 10.1093/hmg/ddv244. Epub 2015 Jun 29. Hum Mol Genet. 2015. PMID: 26123487 Free PMC article.
-
Bone Marrow Mesenchymal Stem Cells Carrying FANCD2 Mutation Differ from the Other Fanconi Anemia Complementation Groups in Terms of TGF-β1 Production.Stem Cell Rev Rep. 2018 Jun;14(3):425-437. doi: 10.1007/s12015-017-9794-5. Stem Cell Rev Rep. 2018. PMID: 29247345
-
Dearth and Delayed Maturation of Testicular Germ Cells in Fanconi Anemia E Mutant Male Mice.PLoS One. 2016 Aug 3;11(8):e0159800. doi: 10.1371/journal.pone.0159800. eCollection 2016. PLoS One. 2016. PMID: 27486799 Free PMC article.
-
The Fanconi anemia ID2 complex: dueling saxes at the crossroads.Cell Cycle. 2014;13(19):2999-3015. doi: 10.4161/15384101.2014.956475. Cell Cycle. 2014. PMID: 25486561 Free PMC article. Review.
-
FANCD2 and DNA Damage.Int J Mol Sci. 2017 Aug 19;18(8):1804. doi: 10.3390/ijms18081804. Int J Mol Sci. 2017. PMID: 28825622 Free PMC article. Review.
Cited by
-
FAAP100 is required for the resolution of transcription-replication conflicts in primordial germ cells.BMC Biol. 2023 Aug 15;21(1):174. doi: 10.1186/s12915-023-01676-1. BMC Biol. 2023. PMID: 37580696 Free PMC article.
-
Global expression pattern of genes containing positively selected sites in European anchovy (Engraulis encrasicolus L.) may shed light on teleost reproduction.PLoS One. 2023 Aug 11;18(8):e0289940. doi: 10.1371/journal.pone.0289940. eCollection 2023. PLoS One. 2023. PMID: 37566603 Free PMC article.
-
LncRNA Landscape of Coronary Atherosclerosis Reveals Differentially Expressed LncRNAs in Proliferation and Migration of Coronary Artery Smooth Muscle Cells.Front Cell Dev Biol. 2021 May 18;9:656636. doi: 10.3389/fcell.2021.656636. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34084771 Free PMC article.
-
Factors Regulating the Activity of LINE1 Retrotransposons.Genes (Basel). 2021 Sep 30;12(10):1562. doi: 10.3390/genes12101562. Genes (Basel). 2021. PMID: 34680956 Free PMC article. Review.
-
A patient with heterochronous double primary tumor of basal ganglia germ cell tumors followed by diffuse hemispheric glioma: a case report.Childs Nerv Syst. 2024 Dec;40(12):4315-4321. doi: 10.1007/s00381-024-06644-w. Epub 2024 Oct 28. Childs Nerv Syst. 2024. PMID: 39466461
References
-
- Agoulnik AI, Lu B, Zhu Q, Truong C, Ty MT, Arango N, Chada KK, Bishop CE 2002. A novel gene, Pog, is necessary for primordial germ cell proliferation in the mouse and underlies the germ cell deficient mutation, gcd. Hum Mol Genet 11 3047–53. - PubMed
-
- Bagby GC 2003. Genetic basis of Fanconi anemia. Curr Opin Hematol 10 68–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

