An organoid biobank for childhood kidney cancers that captures disease and tissue heterogeneity
- PMID: 32161258
- PMCID: PMC7066173
- DOI: 10.1038/s41467-020-15155-6
An organoid biobank for childhood kidney cancers that captures disease and tissue heterogeneity
Abstract
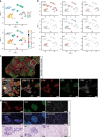
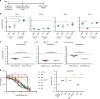
Kidney tumours are among the most common solid tumours in children, comprising distinct subtypes differing in many aspects, including cell-of-origin, genetics, and pathology. Pre-clinical cell models capturing the disease heterogeneity are currently lacking. Here, we describe the first paediatric cancer organoid biobank. It contains tumour and matching normal kidney organoids from over 50 children with different subtypes of kidney cancer, including Wilms tumours, malignant rhabdoid tumours, renal cell carcinomas, and congenital mesoblastic nephromas. Paediatric kidney tumour organoids retain key properties of native tumours, useful for revealing patient-specific drug sensitivities. Using single cell RNA-sequencing and high resolution 3D imaging, we further demonstrate that organoid cultures derived from Wilms tumours consist of multiple different cell types, including epithelial, stromal and blastemal-like cells. Our organoid biobank captures the heterogeneity of paediatric kidney tumours, providing a representative collection of well-characterised models for basic cancer research, drug-screening and personalised medicine.
Conflict of interest statement
J.D. and H.C. are named as inventors on several patents related to leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5)+ stem cell-based organoid technology. The remaining authors declare no competing interests.
Figures




Similar articles
-
The cytological diagnosis of paediatric renal tumours.J Clin Pathol. 2009 Nov;62(11):961-9. doi: 10.1136/jcp.2009.064659. Epub 2009 Aug 20. J Clin Pathol. 2009. PMID: 19700411 Review.
-
Diagnostic utility of Wilms' tumour-1 protein (WT-1) immunostaining in paediatric renal tumours.Indian J Med Res. 2016 May;143(Supplement):S59-S67. doi: 10.4103/0971-5916.191776. Indian J Med Res. 2016. PMID: 27748279 Free PMC article.
-
Results of the SIOP 93-01/GPOH trial and study for the treatment of patients with unilateral nonmetastatic Wilms Tumor.Klin Padiatr. 2004 May-Jun;216(3):132-40. doi: 10.1055/s-2004-822625. Klin Padiatr. 2004. PMID: 15175957 Clinical Trial.
-
[Rare childhood kidney tumors].Pathologe. 2019 Nov;40(6):600-608. doi: 10.1007/s00292-019-0638-8. Pathologe. 2019. PMID: 31338565 Review. German.
-
The establishment of kidney cancer organoid line in drug testing.Cancer Med. 2024 Jun;13(12):e7432. doi: 10.1002/cam4.7432. Cancer Med. 2024. PMID: 38923304 Free PMC article.
Cited by
-
Current Status and Perspectives of Patient-Derived Models for Ewing's Sarcoma.Cancers (Basel). 2020 Sep 4;12(9):2520. doi: 10.3390/cancers12092520. Cancers (Basel). 2020. PMID: 32899796 Free PMC article.
-
MYCN and MAX alterations in Wilms tumor and identification of novel N-MYC interaction partners as biomarker candidates.Cancer Cell Int. 2021 Oct 24;21(1):555. doi: 10.1186/s12935-021-02259-2. Cancer Cell Int. 2021. PMID: 34689785 Free PMC article.
-
Exploring New Dimensions of Tumor Heterogeneity: The Application of Single Cell Analysis to Organoid-Based 3D In Vitro Models.Adv Healthc Mater. 2023 Oct;12(26):e2300903. doi: 10.1002/adhm.202300903. Epub 2023 Aug 27. Adv Healthc Mater. 2023. PMID: 37589373 Free PMC article. Review.
-
The role of organoids in cancer research.Exp Hematol Oncol. 2023 Aug 3;12(1):69. doi: 10.1186/s40164-023-00433-y. Exp Hematol Oncol. 2023. PMID: 37537666 Free PMC article. Review.
-
Tumor Organoid as a Drug Screening Platform for Cancer Research.Curr Stem Cell Res Ther. 2024;19(9):1210-1250. doi: 10.2174/011574888X268366230922080423. Curr Stem Cell Res Ther. 2024. PMID: 37855289 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

