Oestrogen-activated autophagy has a negative effect on the anti-osteoclastogenic function of oestrogen
- PMID: 32157750
- PMCID: PMC7162800
- DOI: 10.1111/cpr.12789
Oestrogen-activated autophagy has a negative effect on the anti-osteoclastogenic function of oestrogen
Erratum in
-
Correction to "Oestrogen-activated autophagy has a negative effect on the anti-osteoclastogenic function of oestrogen".Cell Prolif. 2024 Feb;57(2):ecpr13571. doi: 10.1111/cpr.13571. Epub 2024 Jan 3. Cell Prolif. 2024. PMID: 38173076 Free PMC article. No abstract available.
Abstract
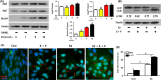
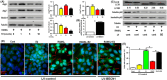
Objectives: Oestrogen is known to inhibit osteoclastogenesis, and numerous studies have identified it as an autophagic activator. To date, the role of oestrogen in the autophagy of osteoclast precursors (OCPs) during osteoclastogenesis remains unclear. This study aimed to determine the effect of autophagy regulated by the biologically active form of oestrogen (17β-estradiol) on osteoclastogenesis.
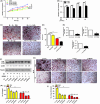
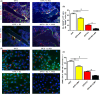
Materials and methods: After treatment with 17β-estradiol in OCPs (from bone marrow-derived macrophages, BMMs) and ovariectomy (OVX) mice, we measured the effect of 17β-estradiol on the autophagy of OCPs in vitro and in vivo. In addition, we studied the role of autophagy in the OCP proliferation, osteoclast differentiation and bone loss regulated by 17β-estradiol using autophagic inhibitor or knock-down of autophagic genes.
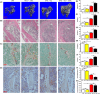
Results: The results showed that direct administration of 17β-estradiol enhanced the autophagic response of OCPs. Interestingly, 17β-estradiol inhibited the stimulatory effect of receptor activator of nuclear factor-κB ligand (RANKL) on the autophagy and osteoclastogenesis of OCPs. Moreover, 17β-estradiol inhibited the downstream signalling of RANKL. Autophagic suppression by pharmacological inhibitors or gene silencing enhanced the inhibitory effect of 17β-estradiol on osteoclastogenesis. In vivo assays showed that the autophagic inhibitor 3-MA not only inhibited the autophagic activity of the OCPs in the trabecular bone of OVX mice but also enhanced the ability of 17β-estradiol to ameliorate bone loss.
Conclusions: In conclusion, our study showed that oestrogen directly enhanced the autophagy of OCPs, which inhibited its anti-osteoclastogenic effect. Drugs based on autophagic inhibition may enhance the efficacy of oestrogen on osteoporosis.
Keywords: LC3; RANKL; autophagy; oestrogen; osteoclastogenesis.
© 2020 The Authors. Cell Proliferation Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no competing interests to disclose.
Figures

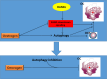
 promote;
promote;  inhibit;
inhibit;  synergy
synergySimilar articles
-
Puerarin inhibits the osteoclastogenesis by inhibiting RANKL-dependent and -independent autophagic responses.BMC Complement Altern Med. 2019 Oct 15;19(1):269. doi: 10.1186/s12906-019-2691-5. BMC Complement Altern Med. 2019. PMID: 31615565 Free PMC article.
-
Curcumin-activated autophagy plays a negative role in its anti-osteoclastogenic effect.Mol Cell Endocrinol. 2020 Jan 15;500:110637. doi: 10.1016/j.mce.2019.110637. Epub 2019 Oct 31. Mol Cell Endocrinol. 2020. PMID: 31678610
-
1α,25-(OH)2-D3 promotes the autophagy during osteoclastogenesis by enhancing RANKL-RANK-TRAF6 signaling.In Vitro Cell Dev Biol Anim. 2021 Oct;57(9):878-885. doi: 10.1007/s11626-021-00632-z. Epub 2021 Nov 15. In Vitro Cell Dev Biol Anim. 2021. PMID: 34780049
-
Autophagy exerts pivotal roles in regulatory effects of 1α,25-(OH)2D3 on the osteoclastogenesis.Biochem Biophys Res Commun. 2019 Apr 16;511(4):869-874. doi: 10.1016/j.bbrc.2019.02.114. Epub 2019 Mar 5. Biochem Biophys Res Commun. 2019. PMID: 30850163
-
Sex-specific lung diseases: effect of oestrogen on cultured cells and in animal models.Eur Respir Rev. 2013 Sep 1;22(129):302-11. doi: 10.1183/09059180.00002813. Eur Respir Rev. 2013. PMID: 23997058 Free PMC article. Review.
Cited by
-
Phosphorylation of BCL2 at the Ser70 site mediates RANKL-induced osteoclast precursor autophagy and osteoclastogenesis.Mol Med. 2022 Feb 19;28(1):22. doi: 10.1186/s10020-022-00449-w. Mol Med. 2022. PMID: 35183115 Free PMC article.
-
Rubiadin-1-methyl ether inhibits BECN1 transcription and Beclin1-dependent autophagy during osteoclastogenesis by inhibiting NF-κB p65 activation.Exp Biol Med (Maywood). 2023 Sep;248(17):1518-1526. doi: 10.1177/15353702231198071. Epub 2023 Sep 26. Exp Biol Med (Maywood). 2023. PMID: 37750211 Free PMC article.
-
Doxorubicin Induces Bone Loss by Increasing Autophagy through a Mitochondrial ROS/TRPML1/TFEB Axis in Osteoclasts.Antioxidants (Basel). 2022 Jul 28;11(8):1476. doi: 10.3390/antiox11081476. Antioxidants (Basel). 2022. PMID: 36009195 Free PMC article.
-
Endogenous Aβ induces osteoporosis through an mTOR-dependent inhibition of autophagy in bone marrow mesenchymal stem cells (BMSCs).Ann Transl Med. 2021 Dec;9(24):1794. doi: 10.21037/atm-21-6427. Ann Transl Med. 2021. PMID: 35071488 Free PMC article.
-
Morroniside inhibits Beclin1-dependent autophagic death and Bax-dependent apoptosis in cardiomyocytes through repressing BCL2 phosphorylation.In Vitro Cell Dev Biol Anim. 2023 Apr;59(4):277-288. doi: 10.1007/s11626-023-00768-0. Epub 2023 May 8. In Vitro Cell Dev Biol Anim. 2023. PMID: 37155079
References
-
- Cappariello A, Maurizi A, Veeriah V, Teti A. The Great Beauty of the osteoclast. Arch Biochem Biophys. 2014;558:70‐78. - PubMed
-
- Christiansen C, Christensen MS, Larsen NE, Transbøl IB. Pathophysiological mechanisms of estrogen effect on bone metabolism, dose‐response relationships in early postmenopausal women. J Clin Endocrinol Metab. 1982;55:1124‐1130. - PubMed
-
- Lindsay R, Hart DM, Forrest C, Baird C. Prevention of spinal osteoporosis in oophorectomized women. Lancet. 1980;2:1151‐1153. - PubMed
-
- Reid DM, Purdie DW, Stevenson JC, Kanis JA, Christiansen C, Kirkman R. Bone density measurements. Lancet. 1992;339:370‐371. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

