The O-GlcNAc Modification on Kinases
- PMID: 32155042
- PMCID: PMC7253032
- DOI: 10.1021/acschembio.9b01015
The O-GlcNAc Modification on Kinases
Abstract
O-Linked N-acetyl glucosamine (O-GlcNAc) is a protein modification found on thousands of nuclear, cytosolic, and mitochondrial proteins. Many O-GlcNAc sites occur in proximity to protein sites that are likewise modified by phosphorylation. While several studies have uncovered crosstalk between these two signaling modifications on individual proteins and pathways, an understanding of the role of O-GlcNAc in regulating kinases, the enzymes that install the phosphate modification, is still emerging. Here we review recent methods to profile the O-GlcNAc modification on a global scale that have revealed more than 100 kinases are modified by O-GlcNAc and highlight existing studies about regulation of these kinases by O-GlcNAc. Continuing efforts to profile the O-GlcNAc proteome and understand the role of O-GlcNAc on kinases will reveal new mechanisms of regulation and potential avenues for manipulation of the signaling mechanisms at the intersection of O-GlcNAc and phosphorylation.
Figures
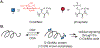




Similar articles
-
O-GlcNAc turns twenty: functional implications for post-translational modification of nuclear and cytosolic proteins with a sugar.FEBS Lett. 2003 Jul 3;546(1):154-8. doi: 10.1016/s0014-5793(03)00641-0. FEBS Lett. 2003. PMID: 12829252 Review.
-
Identification of O-linked N-acetylglucosamine (O-GlcNAc)-modified osteoblast proteins by electron transfer dissociation tandem mass spectrometry reveals proteins critical for bone formation.Mol Cell Proteomics. 2013 Apr;12(4):945-55. doi: 10.1074/mcp.M112.026633. Epub 2013 Feb 26. Mol Cell Proteomics. 2013. PMID: 23443134 Free PMC article.
-
Probing the dynamics of O-GlcNAc glycosylation in the brain using quantitative proteomics.Nat Chem Biol. 2007 Jun;3(6):339-48. doi: 10.1038/nchembio881. Epub 2007 May 13. Nat Chem Biol. 2007. PMID: 17496889
-
Mapping sites of O-GlcNAc modification using affinity tags for serine and threonine post-translational modifications.Mol Cell Proteomics. 2002 Oct;1(10):791-804. doi: 10.1074/mcp.m200048-mcp200. Mol Cell Proteomics. 2002. PMID: 12438562
-
Chemical Reporters and Their Bioorthogonal Reactions for Labeling Protein O-GlcNAcylation.Molecules. 2018 Sep 20;23(10):2411. doi: 10.3390/molecules23102411. Molecules. 2018. PMID: 30241321 Free PMC article. Review.
Cited by
-
Genetically Encoded Boronolectin as a Specific Red Fluorescent UDP-GlcNAc Biosensor.ACS Sens. 2023 Aug 25;8(8):2996-3003. doi: 10.1021/acssensors.3c00409. Epub 2023 Jul 22. ACS Sens. 2023. PMID: 37480329 Free PMC article.
-
Posttranslational Modifications of α-Synuclein, Their Therapeutic Potential, and Crosstalk in Health and Neurodegenerative Diseases.Pharmacol Rev. 2024 Oct 16;76(6):1254-1290. doi: 10.1124/pharmrev.123.001111. Pharmacol Rev. 2024. PMID: 39164116 Free PMC article. Review.
-
The Metabolic Chemical Reporter Ac46AzGal Could Incorporate Intracellular Protein Modification in the Form of UDP-6AzGlc Mediated by OGT and Enzymes in the Leloir Pathway.Front Chem. 2021 Oct 12;9:708306. doi: 10.3389/fchem.2021.708306. eCollection 2021. Front Chem. 2021. PMID: 34712646 Free PMC article.
-
Writing and Erasing O-GlcNAc on Casein Kinase 2 Alpha Alters the Phosphoproteome.ACS Chem Biol. 2022 May 20;17(5):1111-1121. doi: 10.1021/acschembio.1c00987. Epub 2022 Apr 25. ACS Chem Biol. 2022. PMID: 35467332 Free PMC article.
-
Mechanistic roles for altered O-GlcNAcylation in neurodegenerative disorders.Biochem J. 2021 Jul 30;478(14):2733-2758. doi: 10.1042/BCJ20200609. Biochem J. 2021. PMID: 34297044 Free PMC article. Review.
References
-
- Torres CR, and Hart GW (1984) Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc, J Biol Chem 259, 3308–3317. - PubMed
-
- Zachara N, Akimoto Y, and Hart GW (2015) The O-GlcNAc Modification, In Essentials of Glycobiology (Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, and Seeberger PH, Eds.), pp 239–251, Cold Spring Harbor Laboratory Press, Cold Spring Harbor (NY).
-
- Machida M, and Jigami Y (1994) Glycosylated DNA-Binding Proteins from Filamentous Fungus, Aspergillus-Oryzae - Modification with N-Acetylglucosamine Monosaccharide through an O-Glycosidic Linkage, Biosci Biotechn Bioch 58, 344–348.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

