Innate Lymphoid Cells at the Maternal-Fetal Interface in Human Pregnancy
- PMID: 32140065
- PMCID: PMC7053337
- DOI: 10.7150/ijbs.38264
Innate Lymphoid Cells at the Maternal-Fetal Interface in Human Pregnancy
Abstract
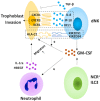
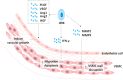
Pregnancy constitutes a major challenge to the maternal immune system, which must tolerate fetal alloantigen encoded by paternal genes. In addition to their role in inducing maternal-fetal immune tolerance, accumulating evidence indicates that decidual immune cells are involved in several processes required for a successful pregnancy, including trophoblast invasion as well as tissue and spiral artery remodeling. Innate lymphoid cells (ILCs), an important branch of the innate immune system, which has expanded rapidly in recent years, are strong actors in mucosal immunity, tissue homeostasis and metabolism regulation. With the recent identification of ILCs in the human decidua, the role of ILCs at the maternal-fetal interface raises concern. Herein, we review the presence and characterization of ILCs in the human decidua, as well as their function in normal pregnancy and pathological pregnancy, including reproductive failure, preeclampsia and others.
Keywords: innate lymphoid cell; preeclampsia.; pregnancy; reproductive failure; spiral artery remodeling; trophoblast invasion.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
Innate Lymphoid Cells in the Maternal and Fetal Compartments.Front Immunol. 2018 Oct 26;9:2396. doi: 10.3389/fimmu.2018.02396. eCollection 2018. Front Immunol. 2018. PMID: 30416502 Free PMC article. Review.
-
Innate lymphoid cells at the human maternal-fetal interface in spontaneous preterm labor.Am J Reprod Immunol. 2018 Jun;79(6):e12820. doi: 10.1111/aji.12820. Epub 2018 Feb 19. Am J Reprod Immunol. 2018. PMID: 29457302 Free PMC article.
-
A balancing act: mechanisms by which the fetus avoids rejection by the maternal immune system.Reproduction. 2011 Jun;141(6):715-24. doi: 10.1530/REP-10-0360. Epub 2011 Mar 9. Reproduction. 2011. PMID: 21389077 Review.
-
The role of RORγt at maternal-fetal interface during murine pregnancy.Am J Reprod Immunol. 2020 Aug;84(2):e13250. doi: 10.1111/aji.13250. Epub 2020 Jun 18. Am J Reprod Immunol. 2020. PMID: 32314428 Free PMC article.
-
Defective trophoblast invasion underlies fetal growth restriction and preeclampsia-like symptoms in the stroke-prone spontaneously hypertensive rat.Mol Hum Reprod. 2017 Jul 1;23(7):509-519. doi: 10.1093/molehr/gax024. Mol Hum Reprod. 2017. PMID: 28402512
Cited by
-
A Hypoxia-Decidual Macrophage Regulatory Axis in Normal Pregnancy and Spontaneous Miscarriage.Int J Mol Sci. 2024 Sep 8;25(17):9710. doi: 10.3390/ijms25179710. Int J Mol Sci. 2024. PMID: 39273657 Free PMC article. Review.
-
Insights into Early-Pregnancy Mechanisms: Mast Cells and Chymase CMA1 Shape the Phenotype and Modulate the Functionality of Human Trophoblast Cells, Vascular Smooth-Muscle Cells and Endothelial Cells.Cells. 2022 Mar 29;11(7):1158. doi: 10.3390/cells11071158. Cells. 2022. PMID: 35406722 Free PMC article.
-
Evolutionary traits and functional roles of chemokines and their receptors in the male pregnancy of the Syngnathidae.Mar Life Sci Technol. 2023 Nov 22;5(4):500-510. doi: 10.1007/s42995-023-00205-x. eCollection 2023 Nov. Mar Life Sci Technol. 2023. PMID: 38045539 Free PMC article.
-
Oxygen regulates ILC3 antigen presentation potential and pregnancy-related hormone actions.Reprod Biol Endocrinol. 2022 Jul 29;20(1):109. doi: 10.1186/s12958-022-00979-2. Reprod Biol Endocrinol. 2022. PMID: 35906658 Free PMC article.
-
How Do Uterine Natural Killer and Innate Lymphoid Cells Contribute to Successful Pregnancy?Front Immunol. 2021 Jun 21;12:607669. doi: 10.3389/fimmu.2021.607669. eCollection 2021. Front Immunol. 2021. PMID: 34234770 Free PMC article. Review.
References
-
- Zhou JZ, Way SS, Chen K. Immunology of the Uterine and Vaginal Mucosae. Trends Immunol. 2018;39:302–14. - PubMed
-
- Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. 2013;13:23–33. - PubMed
-
- Saito S, Shiozaki A, Nakashima A. et al. The role of the immune system in preeclampsia. Mol Aspects Med. 2007;28:192–209. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

