Control of Akt activity and substrate phosphorylation in cells
- PMID: 32125765
- PMCID: PMC7317883
- DOI: 10.1002/iub.2264
Control of Akt activity and substrate phosphorylation in cells
Abstract
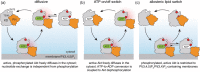
Protein kinase B/Akt is a serine/threonine kinase that links receptors coupled to the PI3K lipid kinase to cellular anabolic pathways. Its activity in cells is controlled by reversible phosphorylation and an intramolecular lipid-controlled allosteric switch. In this review, I outline the current progress in understanding Akt regulatory mechanisms, define three models of Akt activation in cells, and highlight how intramolecular allosterism cooperates with cell-autonomous mechanisms to control Akt localization and activity and direct it toward specific sets of substrates in cells.
Keywords: protein kinase Akt; signaling.
© 2020 The Author. IUBMB Life published by Wiley Periodicals, Inc. on behalf of International Union of Biochemistry and Molecular Biology.
Figures

Similar articles
-
Getting the Akt Together: Guiding Intracellular Akt Activity by PI3K.Biomolecules. 2019 Feb 16;9(2):67. doi: 10.3390/biom9020067. Biomolecules. 2019. PMID: 30781447 Free PMC article. Review.
-
PI(3,4,5)P3 Engagement Restricts Akt Activity to Cellular Membranes.Mol Cell. 2017 Feb 2;65(3):416-431.e6. doi: 10.1016/j.molcel.2016.12.028. Mol Cell. 2017. PMID: 28157504
-
Akt Kinase Activation Mechanisms Revealed Using Protein Semisynthesis.Cell. 2018 Aug 9;174(4):897-907.e14. doi: 10.1016/j.cell.2018.07.003. Epub 2018 Aug 2. Cell. 2018. PMID: 30078705 Free PMC article.
-
Alternative Activation Mechanisms of Protein Kinase B Trigger Distinct Downstream Signaling Responses.J Biol Chem. 2015 Oct 9;290(41):24975-85. doi: 10.1074/jbc.M115.651570. Epub 2015 Aug 18. J Biol Chem. 2015. PMID: 26286748 Free PMC article.
-
Akt/PKB: one kinase, many modifications.Biochem J. 2015 Jun 1;468(2):203-14. doi: 10.1042/BJ20150041. Biochem J. 2015. PMID: 25997832 Review.
Cited by
-
Role of Posttranslational Modifications of Proteins in Cardiovascular Disease.Oxid Med Cell Longev. 2022 Jul 9;2022:3137329. doi: 10.1155/2022/3137329. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35855865 Free PMC article. Review.
-
Inhibitors of phosphoinositide 3-kinase (PI3K) and phosphoinositide 3-kinase-related protein kinase family (PIKK).J Enzyme Inhib Med Chem. 2023 Dec;38(1):2237209. doi: 10.1080/14756366.2023.2237209. J Enzyme Inhib Med Chem. 2023. PMID: 37489050 Free PMC article. Review.
-
NPM promotes hepatotoxin-induced fibrosis by inhibiting ROS-induced apoptosis of hepatic stellate cells and upregulating lncMIAT-induced TGF-β2.Cell Death Dis. 2023 Aug 30;14(8):575. doi: 10.1038/s41419-023-06043-0. Cell Death Dis. 2023. PMID: 37648688 Free PMC article.
-
Protein O-GlcNAcylation in Metabolic Modulation of Skeletal Muscle: A Bright but Long Way to Go.Metabolites. 2022 Sep 22;12(10):888. doi: 10.3390/metabo12100888. Metabolites. 2022. PMID: 36295790 Free PMC article. Review.
-
CircAMOTL1 RNA and AMOTL1 Protein: Complex Functions of AMOTL1 Gene Products.Int J Mol Sci. 2023 Jan 20;24(3):2103. doi: 10.3390/ijms24032103. Int J Mol Sci. 2023. PMID: 36768425 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

