Cellular Senescence in Neurodegenerative Diseases
- PMID: 32116562
- PMCID: PMC7026683
- DOI: 10.3389/fncel.2020.00016
Cellular Senescence in Neurodegenerative Diseases
Abstract
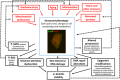
Cellular senescence is a homeostatic biological process characterized by a permanent state of cell cycle arrest that can contribute to the decline of the regenerative potential and function of tissues. The increased presence of senescent cells in different neurodegenerative diseases suggests the contribution of senescence in the pathophysiology of these disorders. Although several factors can induce senescence, DNA damage, oxidative stress, neuroinflammation, and altered proteostasis have been shown to play a role in its onset. Oxidative stress contributes to accelerated aging and cognitive dysfunction stages affecting neurogenesis, neuronal differentiation, connectivity, and survival. During later life stages, it is implicated in the progression of cognitive decline, synapse loss, and neuronal degeneration. Also, neuroinflammation exacerbates oxidative stress, synaptic dysfunction, and neuronal death through the harmful effects of pro-inflammatory cytokines on cell proliferation and maturation. Both oxidative stress and neuroinflammation can induce DNA damage and alterations in DNA repair that, in turn, can exacerbate them. Another important feature associated with senescence is altered proteostasis. Because of the disruption in the function and balance of the proteome, senescence can modify the proper synthesis, folding, quality control, and degradation rate of proteins producing, in some diseases, misfolded proteins or aggregation of abnormal proteins. There is an extensive body of literature that associates cellular senescence with several neurodegenerative disorders including Alzheimer's disease (AD), Down syndrome (DS), and Parkinson's disease (PD). This review summarizes the evidence of the shared neuropathological events in these neurodegenerative diseases and the implication of cellular senescence in their onset or aggravation. Understanding the role that cellular senescence plays in them could help to develop new therapeutic strategies.
Keywords: Alzheimer’s disease; Down syndrome; Parkinsion’s disease; neurodegenaration; senescence.
Copyright © 2020 Martínez-Cué and Rueda.
Figures
Similar articles
-
Proteostasis disruption and senescence in Alzheimer's disease pathways to neurodegeneration.Brain Res. 2024 Dec 15;1845:149202. doi: 10.1016/j.brainres.2024.149202. Epub 2024 Aug 30. Brain Res. 2024. PMID: 39216694 Review.
-
Senescence: A DNA damage response and its role in aging and Neurodegenerative Diseases.Front Aging. 2024 Mar 21;4:1292053. doi: 10.3389/fragi.2023.1292053. eCollection 2023. Front Aging. 2024. PMID: 38596783 Free PMC article. Review.
-
Anti-IL17 treatment ameliorates Down syndrome phenotypes in mice.Brain Behav Immun. 2018 Oct;73:235-251. doi: 10.1016/j.bbi.2018.05.008. Epub 2018 May 31. Brain Behav Immun. 2018. PMID: 29758264
-
Cellular senescence in the aging brain: A promising target for neurodegenerative diseases.Mech Ageing Dev. 2022 Jun;204:111675. doi: 10.1016/j.mad.2022.111675. Epub 2022 Apr 14. Mech Ageing Dev. 2022. PMID: 35430158 Review.
-
Proteostasis Failure in Neurodegenerative Diseases: Focus on Oxidative Stress.Oxid Med Cell Longev. 2020 Mar 27;2020:5497046. doi: 10.1155/2020/5497046. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32308803 Free PMC article. Review.
Cited by
-
A Comprehensive Analysis of the Role of hnRNP A1 Function and Dysfunction in the Pathogenesis of Neurodegenerative Disease.Front Mol Biosci. 2021 Apr 12;8:659610. doi: 10.3389/fmolb.2021.659610. eCollection 2021. Front Mol Biosci. 2021. PMID: 33912591 Free PMC article. Review.
-
Proline Metabolism in Neurological and Psychiatric Disorders.Mol Cells. 2022 Nov 30;45(11):781-788. doi: 10.14348/molcells.2022.0115. Epub 2022 Nov 2. Mol Cells. 2022. PMID: 36324271 Free PMC article. Review.
-
Loss of lamin-B1 and defective nuclear morphology are hallmarks of astrocyte senescence in vitro and in the aging human hippocampus.Aging Cell. 2022 Jan;21(1):e13521. doi: 10.1111/acel.13521. Epub 2021 Dec 10. Aging Cell. 2022. PMID: 34894056 Free PMC article.
-
Normal and Pathological NRF2 Signalling in the Central Nervous System.Antioxidants (Basel). 2022 Jul 22;11(8):1426. doi: 10.3390/antiox11081426. Antioxidants (Basel). 2022. PMID: 35892629 Free PMC article. Review.
-
Biochemical Pathways of Cellular Mechanosensing/Mechanotransduction and Their Role in Neurodegenerative Diseases Pathogenesis.Cells. 2022 Oct 1;11(19):3093. doi: 10.3390/cells11193093. Cells. 2022. PMID: 36231055 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

