The Kaposi's Sarcoma-Associated Herpesvirus (KSHV) gH/gL Complex Is the Predominant Neutralizing Antigenic Determinant in KSHV-Infected Individuals
- PMID: 32111001
- PMCID: PMC7150787
- DOI: 10.3390/v12030256
The Kaposi's Sarcoma-Associated Herpesvirus (KSHV) gH/gL Complex Is the Predominant Neutralizing Antigenic Determinant in KSHV-Infected Individuals
Abstract
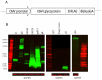
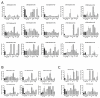
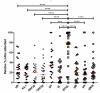
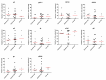
Kaposi's sarcoma-associated herpesvirus (KSHV) is the etiological agent of Kaposi's sarcoma (KS), one of the most prevalent cancers of people living with HIV/AIDS in sub-Saharan Africa. The seroprevalence for KSHV is high in the region, and no prophylactic vaccine against the virus is available. In this study, we characterized the antigenic targets of KSHV-specific neutralizing antibodies (nAbs) in asymptomatic KSHV-infected individuals and KS patients with high nAbs titers. We quantified the extent to which various KSHV envelope glycoproteins (gB, ORF28, ORF68, gH, gL, gM, gN and gpK8.1) adsorbed/removed KSHV-specific nAbs from the plasma of infected individuals. Our study revealed that plasma from a majority of KSHV neutralizers recognizes multiple viral glycoproteins. Moreover, the breadth of nAbs responses against these viral glycoproteins varies among endemic KS, epidemic KS and asymptomatic KSHV-infected individuals. Importantly, among the KSHV glycoproteins, the gH/gL complex, but neither gH nor gL alone, showed the highest adsorption of KSHV-specific nAbs. This activity was detected in 80% of the KSHV-infected individuals regardless of their KS status. The findings suggest that the gH/gL complex is the predominant antigenic determinant of KSHV-specific nAbs. Therefore, gH/gL is a potential target for development of KSHV prophylactic vaccines.
Keywords: KS; KSHV; gH; gL; glycoproteins; neutralizing antibodies.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
BALB/c mice immunized with a combination of virus-like particles incorporating Kaposi sarcoma-associated herpesvirus (KSHV) envelope glycoproteins gpK8.1, gB, and gH/gL induced comparable serum neutralizing antibody activity to UV-inactivated KSHV.Oncotarget. 2017 May 23;8(21):34481-34497. doi: 10.18632/oncotarget.15605. Oncotarget. 2017. PMID: 28404899 Free PMC article.
-
Increased human herpesvirus-8 neutralizing response during remission from Kaposi's sarcoma.J Gen Virol. 2024 Nov;105(11). doi: 10.1099/jgv.0.002044. J Gen Virol. 2024. PMID: 39565219
-
Longitudinal analysis of the humoral response to Kaposi's sarcoma-associated herpesvirus after primary infection in children.J Med Virol. 2016 Nov;88(11):1973-81. doi: 10.1002/jmv.24546. Epub 2016 May 11. J Med Virol. 2016. PMID: 27062052
-
Viral latent proteins as targets for Kaposi's sarcoma and Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) induced lymphoma.Curr Drug Targets Infect Disord. 2003 Jun;3(2):129-35. doi: 10.2174/1568005033481150. Curr Drug Targets Infect Disord. 2003. PMID: 12769790 Review.
-
The Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) in Kaposi's sarcoma, malignant lymphoma, and other diseases.Ann Oncol. 1997;8 Suppl 2:123-9. Ann Oncol. 1997. PMID: 9209655 Review.
Cited by
-
Molecular Virology of KSHV in the Lymphocyte Compartment-Insights From Patient Samples and De Novo Infection Models.Front Cell Infect Microbiol. 2020 Dec 4;10:607663. doi: 10.3389/fcimb.2020.607663. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33344267 Free PMC article. Review.
-
Kaposi's Sarcoma-Associated Herpesvirus, the Etiological Agent of All Epidemiological Forms of Kaposi's Sarcoma.Cancers (Basel). 2021 Dec 9;13(24):6208. doi: 10.3390/cancers13246208. Cancers (Basel). 2021. PMID: 34944828 Free PMC article. Review.
-
Epstein-Barr Virus, But Not Human Papillomavirus, Is Associated With Preinvasive and Invasive Ocular Surface Squamous Neoplasias in Zambian Patients.Front Oncol. 2022 Apr 14;12:864066. doi: 10.3389/fonc.2022.864066. eCollection 2022. Front Oncol. 2022. PMID: 35494029 Free PMC article.
-
Kaposi sarcoma-associated herpesvirus complement control protein (KCP) and glycoprotein K8.1 are not required for viral infection in vitro or in vivo.J Virol. 2024 Jun 13;98(6):e0057624. doi: 10.1128/jvi.00576-24. Epub 2024 May 20. J Virol. 2024. PMID: 38767375 Free PMC article.
-
Antibody profiling and predictive modeling discriminate between Kaposi sarcoma and asymptomatic KSHV infection.PLoS Pathog. 2024 Feb 21;20(2):e1012023. doi: 10.1371/journal.ppat.1012023. eCollection 2024 Feb. PLoS Pathog. 2024. PMID: 38381773 Free PMC article.
References
-
- Blumenthal M.J., Schutz C., Barr D., Locketz M., Marshall V., Whitby D., Katz A.A., Uldrick T., Meintjes G., Schafer G. The Contribution of Kaposi’s Sarcoma-Associated Herpesvirus to Mortality in Hospitalized Human Immunodeficiency Virus-Infected Patients Being Investigated for Tuberculosis in South Africa. J. Infect. Dis. 2019;220:841–851. doi: 10.1093/infdis/jiz180. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

