HIF-2α regulates CD44 to promote cancer stem cell activation in triple-negative breast cancer via PI3K/AKT/mTOR signaling
- PMID: 32110277
- PMCID: PMC7031759
- DOI: 10.4252/wjsc.v12.i1.87
HIF-2α regulates CD44 to promote cancer stem cell activation in triple-negative breast cancer via PI3K/AKT/mTOR signaling
Abstract
Background: Breast cancer is a common malignant tumor that seriously threatens women's health. Breast cancer stem cell (CSC)-like cell population may be the main factor for breast cancer metastasis. Therefore, targeted therapy for CSCs has great potential significance. Hypoxia-inducible factor is a transcription factor widely expressed in tumors. Studies have shown that down-regulation of the hypoxia signaling pathway inhibits tumor stem cell self-renewal and increases the sensitivity of stem cells to radiotherapy and chemotherapy mediated by hypoxia-inducible factor-2α (HIF-2α). However, the specific mechanism remains unclear and further research is necessary.
Aim: To investigate the effect of HIF-2α down-regulation on stem cell markers, microsphere formation, and apoptosis in breast cancer cell line MDA-MB-231 under hypoxia and its possible mechanism.
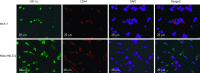
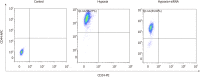
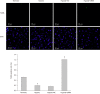
Methods: Immunohistochemistry was used to detect the expression of HIF-2α and CD44 in triple-negative breast cancer (TNBC) and non-TNBC tissues. Double-labeling immunofluorescence was applied to detect the co-expression of HIF-2α and CD44 in MDA-MB-231 cells and MCF-7 cells. HIF-2α was silenced by RNA interference, and the expression of CD44 and transfection efficiency were detected by real-time fluorescent quantitative PCR. Further, flow cytometry, TdT-mediated X-dUTP nick end labeling, and mammosphere formation assays were used to evaluate the effect of HIF-2α on CSCs and apoptosis. The possible mechanisms were analyzed by Western blot.
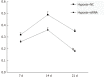
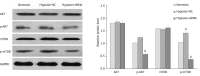
Results: The results of immunohistochemistry showed that HIF-2α was highly expressed in both TNBC and non-TNBC, while the expression of CD44 in different molecular types of breast cancer cells was different. In in vitro experiments, it was found that HIF-2α and CD44 were expressed almost in the same cell. Compared with hypoxia + negative-sequence control, HIF-2α small interfering ribonucleic acid transfection can lower the expression of HIF-2α and CD44 mRNA(P < 0.05), increase the percentage of apoptotic cells (P < 0.05), and resulted in a reduction of CD44+/CD24- population (P < 0.05) and mammosphere formation (P < 0.05) in hypoxic MDA-MB-231 cells. Western blot analysis revealed that phosphorylated protein-serine-threonine kinase (p-AKT) and phosphorylated mammalian target of rapamycin (p-mTOR) levels in MDA-MB-231 decreased significantly after HIF-2α silencing (P < 0.05).
Conclusion: Down-regulation of HIF-2α expression can inhibit the stemness of human breast cancer MDA-MB-231 cells and promote apoptosis, and its mechanism may be related to the CD44/phosphoinosmde-3-kinase/AKT/mTOR signaling pathway.
Keywords: Breast cancer; CD44; Cancer stem cells; Hypoxia-inducible factor-2α.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors report no conflicts of interest in this work.
Figures

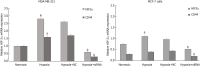
Similar articles
-
[Silencing hypoxia inducible factor-2α gene by small interference RNA inhibits the growth of mammosphere cells in nude mice under hypoxic microenvironment].Zhonghua Yi Xue Za Zhi. 2013 Apr 16;93(15):1182-7. Zhonghua Yi Xue Za Zhi. 2013. PMID: 23902893 Chinese.
-
[Effect of HIF-2α on the biological characteristics of breast cancer stem cells].Zhonghua Yi Xue Za Zhi. 2018 Jan 23;98(4):269-273. doi: 10.3760/cma.j.issn.0376-2491.2018.04.006. Zhonghua Yi Xue Za Zhi. 2018. PMID: 29397612 Chinese.
-
A recombinant adenovirus vector containing the synNotch receptor gene for the treatment of triple-negative breast cancer.Front Oncol. 2023 Mar 29;13:1147668. doi: 10.3389/fonc.2023.1147668. eCollection 2023. Front Oncol. 2023. PMID: 37064130 Free PMC article.
-
A novel cross-communication of HIF-1α and HIF-2α with Wnt signaling in TNBC and influence of hypoxic microenvironment in the formation of an organ-on-chip model of breast cancer.Med Oncol. 2023 Jul 15;40(8):245. doi: 10.1007/s12032-023-02112-8. Med Oncol. 2023. PMID: 37454033 Review.
-
Hypoxia: syndicating triple negative breast cancer against various therapeutic regimens.Front Oncol. 2023 Jul 10;13:1199105. doi: 10.3389/fonc.2023.1199105. eCollection 2023. Front Oncol. 2023. PMID: 37492478 Free PMC article. Review.
Cited by
-
A novel HIF-2α targeted inhibitor suppresses hypoxia-induced breast cancer stemness via SOD2-mtROS-PDI/GPR78-UPRER axis.Cell Death Differ. 2022 Sep;29(9):1769-1789. doi: 10.1038/s41418-022-00963-8. Epub 2022 Mar 17. Cell Death Differ. 2022. PMID: 35301432 Free PMC article.
-
Cancer Stem Cell for Tumor Therapy.Cancers (Basel). 2021 Sep 26;13(19):4814. doi: 10.3390/cancers13194814. Cancers (Basel). 2021. PMID: 34638298 Free PMC article. Review.
-
MiR-526b-3p Attenuates Breast Cancer Stem Cell Properties and Chemoresistance by Targeting HIF-2α/Notch Signaling.Front Oncol. 2021 Dec 23;11:696269. doi: 10.3389/fonc.2021.696269. eCollection 2021. Front Oncol. 2021. PMID: 35004266 Free PMC article.
-
Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments.Mol Cancer. 2021 Jan 4;20(1):7. doi: 10.1186/s12943-020-01288-1. Mol Cancer. 2021. PMID: 33397409 Free PMC article. Review.
-
Novel Approaches with HIF-2α Targeted Therapies in Metastatic Renal Cell Carcinoma.Cancers (Basel). 2024 Jan 31;16(3):601. doi: 10.3390/cancers16030601. Cancers (Basel). 2024. PMID: 38339352 Free PMC article. Review.
References
-
- Templeton AJ, Ace O, McNamara MG, Al-Mubarak M, Vera-Badillo FE, Hermanns T, Seruga B, Ocaña A, Tannock IF, Amir E. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23:1204–1212. - PubMed
-
- Neil-Sztramko SE, Winters-Stone KM, Bland KA, Campbell KL. Updated systematic review of exercise studies in breast cancer survivors: attention to the principles of exercise training. Br J Sports Med. 2019;53:504–512. - PubMed
-
- Brandão T, Schulz MS, Matos PM. Psychological adjustment after breast cancer: a systematic review of longitudinal studies. Psychooncology. 2017;26:917–926. - PubMed
-
- Ennour-Idrissi K, Maunsell E, Diorio C. Telomere Length and Breast Cancer Prognosis: A Systematic Review. Cancer Epidemiol Biomarkers Prev. 2017;26:3–10. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

