Histone demethylase KDM3B protects against ferroptosis by upregulating SLC7A11
- PMID: 32107878
- PMCID: PMC7137800
- DOI: 10.1002/2211-5463.12823
Histone demethylase KDM3B protects against ferroptosis by upregulating SLC7A11
Abstract
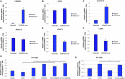
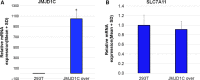
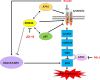
Ferroptosis is a type of adaptive cell death driven by cellular metabolism and iron-dependent lipid peroxidation. Though multiple genes (including SLC7A11 and GPX4) have been demonstrated to play key roles in ferroptosis, little is known about the epigenetic regulation of this process. Here, we report that KDM3B, a histone H3 lysine 9 demethylase, can protect against ferroptosis induced by Erastin, an inhibitor of SLC7A11. KDM3B overexpression in HT-1080 cells results in decreased histone H3 lysine 9 methylation. Furthermore, KDM3B upregulates the expression of SLC7A11 through cooperation with the transcription factor ATF4. In summary, we identify here KDM3B as a potential epigenetic regulator of ferroptosis.
Keywords: ATF4; KDM3B; SLC7A11; ferroptosis; histone; histone demethylase.
© 2020 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
All authors declare no conflict of interest.
Figures

Similar articles
-
Regulation of H2A ubiquitination and SLC7A11 expression by BAP1 and PRC1.Cell Cycle. 2019 Apr;18(8):773-783. doi: 10.1080/15384101.2019.1597506. Epub 2019 Mar 30. Cell Cycle. 2019. PMID: 30907299 Free PMC article.
-
KDM4A-mediated histone demethylation of SLC7A11 inhibits cell ferroptosis in osteosarcoma.Biochem Biophys Res Commun. 2021 Apr 23;550:77-83. doi: 10.1016/j.bbrc.2021.02.137. Epub 2021 Mar 6. Biochem Biophys Res Commun. 2021. PMID: 33689883
-
Lipid Peroxidation, GSH Depletion, and SLC7A11 Inhibition Are Common Causes of EMT and Ferroptosis in A549 Cells, but Different in Specific Mechanisms.DNA Cell Biol. 2021 Feb;40(2):172-183. doi: 10.1089/dna.2020.5730. Epub 2020 Dec 22. DNA Cell Biol. 2021. PMID: 33351681
-
Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy.Protein Cell. 2021 Aug;12(8):599-620. doi: 10.1007/s13238-020-00789-5. Epub 2020 Oct 1. Protein Cell. 2021. PMID: 33000412 Free PMC article. Review.
-
Epigenetic gene regulation by plant Jumonji group of histone demethylase.Biochim Biophys Acta. 2011 Aug;1809(8):421-6. doi: 10.1016/j.bbagrm.2011.03.004. Epub 2011 Mar 16. Biochim Biophys Acta. 2011. PMID: 21419882 Review.
Cited by
-
Ferroptosis: an important player in the inflammatory response in diabetic nephropathy.Front Immunol. 2023 Dec 4;14:1294317. doi: 10.3389/fimmu.2023.1294317. eCollection 2023. Front Immunol. 2023. PMID: 38111578 Free PMC article. Review.
-
The Emerging Role of Ferroptosis in Sepsis, Opportunity or Challenge?Infect Drug Resist. 2023 Aug 23;16:5551-5562. doi: 10.2147/IDR.S419993. eCollection 2023. Infect Drug Resist. 2023. PMID: 37641800 Free PMC article. Review.
-
The Organelle-Specific Regulations and Epigenetic Regulators in Ferroptosis.Front Pharmacol. 2022 Jun 17;13:905501. doi: 10.3389/fphar.2022.905501. eCollection 2022. Front Pharmacol. 2022. PMID: 35784729 Free PMC article. Review.
-
Inhibition of LSD1 induces ferroptosis through the ATF4-xCT pathway and shows enhanced anti-tumor effects with ferroptosis inducers in NSCLC.Cell Death Dis. 2023 Nov 3;14(11):716. doi: 10.1038/s41419-023-06238-5. Cell Death Dis. 2023. PMID: 37923740 Free PMC article.
-
System Xc -/GSH/GPX4 axis: An important antioxidant system for the ferroptosis in drug-resistant solid tumor therapy.Front Pharmacol. 2022 Aug 29;13:910292. doi: 10.3389/fphar.2022.910292. eCollection 2022. Front Pharmacol. 2022. PMID: 36105219 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

