Leaky scanning translation generates a second A49 protein that contributes to vaccinia virus virulence
- PMID: 32100702
- PMCID: PMC7414448
- DOI: 10.1099/jgv.0.001386
Leaky scanning translation generates a second A49 protein that contributes to vaccinia virus virulence
Abstract
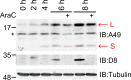
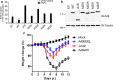
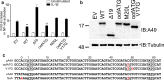
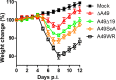
Vaccinia virus (VACV) strain Western Reserve gene A49L encodes a small intracellular protein with a Bcl-2 fold that is expressed early during infection and has multiple functions. A49 co-precipitates with the E3 ubiquitin ligase β-TrCP and thereby prevents ubiquitylation and proteasomal degradation of IκBα, and consequently blocks activation of NF-κB. In a similar way, A49 stabilizes β-catenin, leading to activation of the wnt signalling pathway. However, a VACV strain expressing a mutant A49 that neither co-precipitates with β-TrCP nor inhibits NF-κB activation, is more virulent than a virus lacking A49, indicating that A49 has another function that also contributes to virulence. Here we demonstrate that gene A49L encodes a second, smaller polypeptide that is expressed via leaky scanning translation from methionine 20 and is unable to block NF-κB activation. Viruses engineered to express either only the large protein or only the small A49 protein both have lower virulence than wild-type virus and greater virulence than an A49L deletion mutant. This demonstrates that the small protein contributes to virulence by an unknown mechanism that is independent of NF-κB inhibition. Despite having a large genome with about 200 genes, this study illustrates how VACV makes efficient use of its coding potential and from gene A49L expresses a protein with multiple functions and multiple proteins with different functions.
Keywords: Bcl2-fold; NF-κB inhibitor; gene A49R; leaky scanning; multiple functions; vaccinia virus; virulence.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


Similar articles
-
NF-κB activation is a turn on for vaccinia virus phosphoprotein A49 to turn off NF-κB activation.Proc Natl Acad Sci U S A. 2019 Mar 19;116(12):5699-5704. doi: 10.1073/pnas.1813504116. Epub 2019 Feb 28. Proc Natl Acad Sci U S A. 2019. PMID: 30819886 Free PMC article.
-
Poxvirus targeting of E3 ligase β-TrCP by molecular mimicry: a mechanism to inhibit NF-κB activation and promote immune evasion and virulence.PLoS Pathog. 2013 Feb;9(2):e1003183. doi: 10.1371/journal.ppat.1003183. Epub 2013 Feb 28. PLoS Pathog. 2013. PMID: 23468625 Free PMC article.
-
Vaccinia virus protein A49 is an unexpected member of the B-cell Lymphoma (Bcl)-2 protein family.J Biol Chem. 2015 Mar 6;290(10):5991-6002. doi: 10.1074/jbc.M114.624650. Epub 2015 Jan 20. J Biol Chem. 2015. PMID: 25605733 Free PMC article.
-
Vaccinia Virus BBK E3 Ligase Adaptor A55 Targets Importin-Dependent NF-κB Activation and Inhibits CD8+ T-Cell Memory.J Virol. 2019 May 1;93(10):e00051-19. doi: 10.1128/JVI.00051-19. Print 2019 May 15. J Virol. 2019. PMID: 30814284 Free PMC article.
-
Regulation of NF-κB by ubiquitination and degradation of the IκBs.Immunol Rev. 2012 Mar;246(1):77-94. doi: 10.1111/j.1600-065X.2012.01098.x. Immunol Rev. 2012. PMID: 22435548 Review.
Cited by
-
Progress on Poxvirus E3 Ubiquitin Ligases and Adaptor Proteins.Front Immunol. 2021 Dec 9;12:740223. doi: 10.3389/fimmu.2021.740223. eCollection 2021. Front Immunol. 2021. PMID: 34956175 Free PMC article. Review.
-
Vaccinia Virus Activation and Antagonism of Cytosolic DNA Sensing.Front Immunol. 2020 Oct 1;11:568412. doi: 10.3389/fimmu.2020.568412. eCollection 2020. Front Immunol. 2020. PMID: 33117352 Free PMC article. Review.
-
The actin nucleator Spir-1 is a virus restriction factor that promotes innate immune signalling.PLoS Pathog. 2022 Feb 11;18(2):e1010277. doi: 10.1371/journal.ppat.1010277. eCollection 2022 Feb. PLoS Pathog. 2022. PMID: 35148361 Free PMC article.
References
-
- Moss B. Poxviridae. In: Knipe DMH, editor. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; 2013. pp. 2129–2159. editor.
-
- Mansur DS, Maluquer de Motes C, Unterholzner L, Sumner RP, Ferguson BJ, et al. Poxvirus targeting of E3 ligase β-TrCP by molecular mimicry: a mechanism to inhibit NF-κB activation and promote immune evasion and virulence. PLoS Pathog. 2013;9:e1003183. doi: 10.1371/journal.ppat.1003183. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

