Neuromuscular Diseases Due to Chaperone Mutations: A Review and Some New Results
- PMID: 32093037
- PMCID: PMC7073051
- DOI: 10.3390/ijms21041409
Neuromuscular Diseases Due to Chaperone Mutations: A Review and Some New Results
Abstract
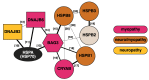
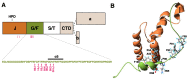
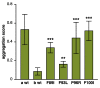
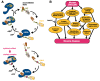
Skeletal muscle and the nervous system depend on efficient protein quality control, and they express chaperones and cochaperones at high levels to maintain protein homeostasis. Mutations in many of these proteins cause neuromuscular diseases, myopathies, and hereditary motor and sensorimotor neuropathies. In this review, we cover mutations in DNAJB6, DNAJB2, αB-crystallin (CRYAB, HSPB5), HSPB1, HSPB3, HSPB8, and BAG3, and discuss the molecular mechanisms by which they cause neuromuscular disease. In addition, previously unpublished results are presented, showing downstream effects of BAG3 p.P209L on DNAJB6 turnover and localization.
Keywords: J-domain protein; heat shock protein; myopathy; neuropathy; pathomechanism.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
HspB8, a small heat shock protein mutated in human neuromuscular disorders, has in vivo chaperone activity in cultured cells.Hum Mol Genet. 2005 Jun 15;14(12):1659-69. doi: 10.1093/hmg/ddi174. Epub 2005 May 6. Hum Mol Genet. 2005. PMID: 15879436
-
Metformin rescues muscle function in BAG3 myofibrillar myopathy models.Autophagy. 2021 Sep;17(9):2494-2510. doi: 10.1080/15548627.2020.1833500. Epub 2020 Oct 19. Autophagy. 2021. PMID: 33030392 Free PMC article.
-
An interaction study in mammalian cells demonstrates weak binding of HSPB2 to BAG3, which is regulated by HSPB3 and abrogated by HSPB8.Cell Stress Chaperones. 2017 Jul;22(4):531-540. doi: 10.1007/s12192-017-0769-x. Epub 2017 Feb 8. Cell Stress Chaperones. 2017. PMID: 28181153 Free PMC article.
-
Hsp27 (HspB1) and alphaB-crystallin (HspB5) as therapeutic targets.FEBS Lett. 2007 Jul 31;581(19):3665-74. doi: 10.1016/j.febslet.2007.04.033. Epub 2007 Apr 24. FEBS Lett. 2007. PMID: 17467701 Review.
-
Small heat shock proteins HSP27 (HspB1), αB-crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death.Int J Biochem Cell Biol. 2012 Oct;44(10):1622-31. doi: 10.1016/j.biocel.2012.04.002. Epub 2012 Apr 13. Int J Biochem Cell Biol. 2012. PMID: 22521623 Review.
Cited by
-
A novel recessive mutation affecting DNAJB6a causes myofibrillar myopathy.Acta Neuropathol Commun. 2021 Feb 8;9(1):23. doi: 10.1186/s40478-020-01046-w. Acta Neuropathol Commun. 2021. PMID: 33557929 Free PMC article.
-
Case Report: A Novel Splice-Site Mutation in DNAJB6 Associated With Juvenile-Onset Proximal-Distal Myopathy in a Chinese Patient.Front Genet. 2022 Jun 23;13:925926. doi: 10.3389/fgene.2022.925926. eCollection 2022. Front Genet. 2022. PMID: 35812750 Free PMC article.
-
DnaJC7 specifically regulates tau seeding.bioRxiv [Preprint]. 2023 Mar 16:2023.03.16.532880. doi: 10.1101/2023.03.16.532880. bioRxiv. 2023. Update in: Elife. 2023 Jun 30;12:e86936. doi: 10.7554/eLife.86936 PMID: 36993367 Free PMC article. Updated. Preprint.
-
Cellular Stress in the Pathogenesis of Muscular Disorders-From Cause to Consequence.Int J Mol Sci. 2020 Aug 13;21(16):5830. doi: 10.3390/ijms21165830. Int J Mol Sci. 2020. PMID: 32823799 Free PMC article. Review.
-
Early onset hereditary neuronopathies: an update on non-5q motor neuron diseases.Brain. 2023 Mar 1;146(3):806-822. doi: 10.1093/brain/awac452. Brain. 2023. PMID: 36445400 Free PMC article. Review.
References
-
- Taipale M., Tucker G., Peng J., Krykbaeva I., Lin Z.Y., Larsen B., Choi H., Berger B., Gingras A.C., Lindquist S. A Quantitative Chaperone Interaction Network Reveals the Architecture of Cellular Protein Homeostasis Pathways. Cell. 2014;158:434–448. doi: 10.1016/j.cell.2014.05.039. - DOI - PMC - PubMed
-
- Carra S., Alberti S., Benesch J.L.P., Boelens W., Buchner J., Carver J.A., Cecconi C., Ecroyd H., Gusev N., Hightower L.E., et al. Small Heat Shock Proteins: Multifaceted Proteins with Important Implications for Life. Cell Stress Chaperones. 2019;24:295–308. doi: 10.1007/s12192-019-00979-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

