Cross-talk signaling in the trigeminal ganglion: role of neuropeptides and other mediators
- PMID: 32088764
- PMCID: PMC7148261
- DOI: 10.1007/s00702-020-02161-7
Cross-talk signaling in the trigeminal ganglion: role of neuropeptides and other mediators
Abstract
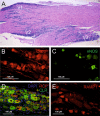
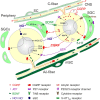
The trigeminal ganglion with its three trigeminal nerve tracts consists mainly of clusters of sensory neurons with their peripheral and central processes. Most neurons are surrounded by satellite glial cells and the axons are wrapped by myelinating and non-myelinating Schwann cells. Trigeminal neurons express various neuropeptides, most notably, calcitonin gene-related peptide (CGRP), substance P, and pituitary adenylate cyclase-activating polypeptide (PACAP). Two types of CGRP receptors are expressed in neurons and satellite glia. A variety of other signal molecules like ATP, nitric oxide, cytokines, and neurotrophic factors are released from trigeminal ganglion neurons and signal to neighboring neurons or satellite glial cells, which can signal back to neurons with same or other mediators. This potential cross-talk of signals involves intracellular mechanisms, including gene expression, that can modulate mediators of sensory information, such as neuropeptides, receptors, and neurotrophic factors. From the ganglia cell bodies, which are outside the blood-brain barrier, the mediators are further distributed to peripheral sites and/or to the spinal trigeminal nucleus in the brainstem, where they can affect neural transmission. A major question is how the sensory neurons in the trigeminal ganglion differ from those in the dorsal root ganglion. Despite their functional overlap, there are distinct differences in their ontogeny, gene expression, signaling pathways, and responses to anti-migraine drugs. Consequently, drugs that modulate cross-talk in the trigeminal ganglion can modulate both peripheral and central sensitization, which may potentially be distinct from sensitization mediated in the dorsal root ganglion.
Keywords: CGRP; Dorsal root ganglion; Neuropeptides; Satellite glial cells; Sensory neurons; Signaling; Trigeminal ganglion.
Figures
Similar articles
-
Current understanding of trigeminal ganglion structure and function in headache.Cephalalgia. 2019 Nov;39(13):1661-1674. doi: 10.1177/0333102418786261. Epub 2018 Jul 10. Cephalalgia. 2019. PMID: 29989427 Free PMC article. Review.
-
Pituitary adenylate cyclase activating polypeptide expression in sensory neurons.Neuroscience. 1994 Nov;63(1):307-12. doi: 10.1016/0306-4522(94)90025-6. Neuroscience. 1994. PMID: 7898655
-
PACAP-38 but not VIP induces release of CGRP from trigeminal nucleus caudalis via a receptor distinct from the PAC1 receptor.Neuropeptides. 2014 Apr;48(2):53-64. doi: 10.1016/j.npep.2014.01.004. Epub 2014 Jan 25. Neuropeptides. 2014. PMID: 24508136
-
Subpopulations of primary sensory neurons show coexistence of neuropeptides and glucocorticoid receptors in the rat spinal and trigeminal ganglia.Brain Res. 1994 Feb 14;636(2):338-42. doi: 10.1016/0006-8993(94)91034-0. Brain Res. 1994. PMID: 8012818
-
CGRP and the Trigeminal System in Migraine.Headache. 2019 May;59(5):659-681. doi: 10.1111/head.13529. Epub 2019 Apr 14. Headache. 2019. PMID: 30982963 Free PMC article. Review.
Cited by
-
Lasmiditan and 5-Hydroxytryptamine in the rat trigeminal system; expression, release and interactions with 5-HT1 receptors.J Headache Pain. 2022 Feb 17;23(1):26. doi: 10.1186/s10194-022-01394-z. J Headache Pain. 2022. PMID: 35177004 Free PMC article.
-
Single-cell transcriptomic profile of satellite glial cells in trigeminal ganglion.Front Mol Neurosci. 2023 Feb 2;16:1117065. doi: 10.3389/fnmol.2023.1117065. eCollection 2023. Front Mol Neurosci. 2023. PMID: 36818656 Free PMC article.
-
Advances in the understanding and treatment of pain and headache.J Neural Transm (Vienna). 2020 Apr;127(4):389-392. doi: 10.1007/s00702-020-02164-4. J Neural Transm (Vienna). 2020. PMID: 32172470 No abstract available.
-
Potent dual MAGL/FAAH inhibitor AKU-005 engages endocannabinoids to diminish meningeal nociception implicated in migraine pain.J Headache Pain. 2023 Apr 11;24(1):38. doi: 10.1186/s10194-023-01568-3. J Headache Pain. 2023. PMID: 37038131 Free PMC article.
-
Ultrasound localization microscopy and functional ultrasound imaging reveal atypical features of the trigeminal ganglion vasculature.Commun Biol. 2022 Apr 7;5(1):330. doi: 10.1038/s42003-022-03273-4. Commun Biol. 2022. PMID: 35393515 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

