Deguelin suppresses non-small cell lung cancer by inhibiting EGFR signaling and promoting GSK3β/FBW7-mediated Mcl-1 destabilization
- PMID: 32081857
- PMCID: PMC7035355
- DOI: 10.1038/s41419-020-2344-0
Deguelin suppresses non-small cell lung cancer by inhibiting EGFR signaling and promoting GSK3β/FBW7-mediated Mcl-1 destabilization
Abstract
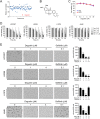
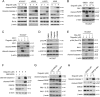
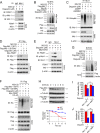
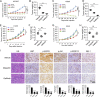
Activating mutations of epidermal growth factor receptor (EGFR) play crucial roles in the oncogenesis of human non-small cell lung cancer (NSCLC). By screening 79 commercially available natural products, we found that the natural compound deguelin exhibited a profound anti-tumor effect on NSCLC via directly down-regulating of EGFR-signaling pathway. Deguelin potently inhibited in vitro EGFR kinase activity of wild type (WT), exon 19 deletion, and L858R/T790M-mutated EGFR. The in silico docking study indicated that deguelin was docked into the ATP-binding pocket of EGFRs. By suppression of EGFR signaling, deguelin inhibited anchorage-dependent, and independent growth of NSCLC cell lines, and significantly delayed tumorigenesis in vivo. Further study showed that deguelin inhibited EGFR and downstream kinase Akt, which resulted in the activation of GSK3β and eventually enhanced Mcl-1 phosphorylation at S159. Moreover, deguelin promoted the interaction between Mcl-1 and E3 ligase SCFFBW7, which enhanced FBW7-mediated Mcl-1 ubiquitination and degradation. Additionally, phosphorylation of Mcl-1 by GSK3β is a prerequisite for FBW7-mediated Mcl-1 destruction. Depletion or pharmacological inactivation of GSK3β compromised deguelin-induced Mcl-1 ubiquitination and reduction. Taken together, our data indicate that enhancement of ubiquitination-dependent Mcl-1 turnover might be a promising approach for cancer treatment.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Formononetin inhibits tumor growth by suppression of EGFR-Akt-Mcl-1 axis in non-small cell lung cancer.J Exp Clin Cancer Res. 2020 Apr 10;39(1):62. doi: 10.1186/s13046-020-01566-2. J Exp Clin Cancer Res. 2020. PMID: 32276600 Free PMC article.
-
MDM2 drives resistance to Osimertinib by contextually disrupting FBW7-mediated destruction of MCL-1 protein in EGFR mutant NSCLC.J Exp Clin Cancer Res. 2024 Nov 15;43(1):302. doi: 10.1186/s13046-024-03220-7. J Exp Clin Cancer Res. 2024. PMID: 39543744 Free PMC article.
-
Targeting FBW7 as a Strategy to Overcome Resistance to Targeted Therapy in Non-Small Cell Lung Cancer.Cancer Res. 2017 Jul 1;77(13):3527-3539. doi: 10.1158/0008-5472.CAN-16-3470. Epub 2017 May 18. Cancer Res. 2017. PMID: 28522751
-
Pharmacological basis and new insights of deguelin concerning its anticancer effects.Pharmacol Res. 2021 Dec;174:105935. doi: 10.1016/j.phrs.2021.105935. Epub 2021 Oct 10. Pharmacol Res. 2021. PMID: 34644595 Review.
-
Mcl-1 ubiquitination and destruction.Oncotarget. 2011 Mar;2(3):239-44. doi: 10.18632/oncotarget.242. Oncotarget. 2011. PMID: 21608150 Free PMC article. Review.
Cited by
-
TRAF4-mediated ubiquitination-dependent activation of JNK/Bcl-xL drives radioresistance.Cell Death Dis. 2023 Feb 10;14(2):102. doi: 10.1038/s41419-023-05637-y. Cell Death Dis. 2023. PMID: 36765039 Free PMC article.
-
Advances of E3 ligases in lung cancer.Biochem Biophys Rep. 2024 May 27;38:101740. doi: 10.1016/j.bbrep.2024.101740. eCollection 2024 Jul. Biochem Biophys Rep. 2024. PMID: 38841185 Free PMC article. Review.
-
Targeting Aurora B kinase with Tanshinone IIA suppresses tumor growth and overcomes radioresistance.Cell Death Dis. 2021 Feb 4;12(2):152. doi: 10.1038/s41419-021-03434-z. Cell Death Dis. 2021. PMID: 33542222 Free PMC article.
-
Pan-EGFR Inhibitor Dacomitinib Resensitizes Paclitaxel and Induces Apoptosis via Elevating Intracellular ROS Levels in Ovarian Cancer SKOV3-TR Cells.Molecules. 2024 Jan 4;29(1):274. doi: 10.3390/molecules29010274. Molecules. 2024. PMID: 38202856 Free PMC article.
-
Deguelin Restores Paclitaxel Sensitivity in Paclitaxel-Resistant Ovarian Cancer Cells via Inhibition of the EGFR Signaling Pathway.Cancer Manag Res. 2024 May 28;16:507-525. doi: 10.2147/CMAR.S457221. eCollection 2024. Cancer Manag Res. 2024. PMID: 38827785 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

