Osteoclastogenesis in periodontal diseases: Possible mediators and mechanisms
- PMID: 32081710
- PMCID: PMC7329621
- DOI: 10.1016/j.job.2020.02.002
Osteoclastogenesis in periodontal diseases: Possible mediators and mechanisms
Abstract
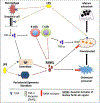
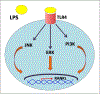
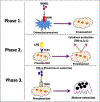
Background: Periodontitis is the inflammation of the tooth-supporting structures and is one of the most common diseases of the oral cavity. The outcome of periodontal infections is tooth loss due to a lack of alveolar bone support. Osteoclasts are giant, multi-nucleated, and bone-resorbing cells that are central for many osteolytic diseases, including periodontitis. Receptor activator of nuclear factor-kB ligand (RANKL) is the principal factor involved in osteoclast differentiation, activation, and survival. However, under pathological conditions, a variety of pro-inflammatory cytokines secreted by activated immune cells also contribute to osteoclast differentiation and activity. Lipopolysaccharide (LPS) is a vital component of the outer membrane of the Gram-negative bacteria. It binds to the Toll-like receptors (TLRs) expressed in many cells and elicits an immune response.
Highlights: The presence of bacterial LPS in the periodontal area stimulates the secretion of RANKL as well as other inflammatory mediators, activating the process of osteoclastogenesis. RANKL, either independently or synergistically with LPS, can regulate osteoclastogenesis, while LPS alone cannot. MicroRNA, IL-22, M1/M2 macrophages, and memory B cells have recently been shown to modulate osteoclastogenesis in periodontal diseases.
Conclusion: In this review, we summarize the mechanism of osteoclastogenesis accompanying periodontal diseases at the cellular level. We discuss a) the effects of LPS/TLR signaling and other cytokines on RANKL-dependent and -independent mechanisms involved in osteoclastogenesis; b) the recently identified role of several endogenous factors such as miRNA, IL-22, M1/M2 macrophages, and memory B cells in regulating osteoclastogenesis during periodontal pathogenesis.
Keywords: Alveolar bone loss; Lipopolysaccharides; Osteoclasts; RANK ligand.
Copyright © 2020 Japanese Association for Oral Biology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflicts of interest The authors have no potential conflict of interest relevant to this article.
Figures
Similar articles
-
Lipopolysaccharide- TLR-4 Axis regulates Osteoclastogenesis independent of RANKL/RANK signaling.BMC Immunol. 2021 Mar 25;22(1):23. doi: 10.1186/s12865-021-00409-9. BMC Immunol. 2021. PMID: 33765924 Free PMC article.
-
Recombinant thrombomodulin lectin-like domain attenuates Porphyromonas gingivalis lipopolysaccharide-induced osteoclastogenesis and periodontal bone resorption.J Periodontol. 2021 Nov;92(11):1622-1634. doi: 10.1002/JPER.20-0732. Epub 2021 Feb 6. J Periodontol. 2021. PMID: 33438207
-
Aminothiazoles inhibit osteoclastogenesis and PGE2 production in LPS-stimulated co-cultures of periodontal ligament and RAW 264.7 cells, and RANKL-mediated osteoclastogenesis and bone resorption in PBMCs.J Cell Mol Med. 2019 Feb;23(2):1152-1163. doi: 10.1111/jcmm.14015. Epub 2018 Dec 1. J Cell Mol Med. 2019. PMID: 30506812 Free PMC article.
-
Immune response: the key to bone resorption in periodontal disease.J Periodontol. 2005 Nov;76(11 Suppl):2033-41. doi: 10.1902/jop.2005.76.11-S.2033. J Periodontol. 2005. PMID: 16277573 Review.
-
Role of periodontal ligament fibroblasts in osteoclastogenesis: a review.J Periodontal Res. 2015 Apr;50(2):152-9. doi: 10.1111/jre.12197. Epub 2014 May 24. J Periodontal Res. 2015. PMID: 24862732 Review.
Cited by
-
SLIT2 Overexpression in Periodontitis Intensifies Inflammation and Alveolar Bone Loss, Possibly via the Activation of MAPK Pathway.Front Cell Dev Biol. 2020 Jul 14;8:593. doi: 10.3389/fcell.2020.00593. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32760720 Free PMC article.
-
Xanthine Derivative KMUP-1 Attenuates Experimental Periodontitis by Reducing Osteoclast Differentiation and Inflammation.Front Pharmacol. 2022 Apr 28;13:821492. doi: 10.3389/fphar.2022.821492. eCollection 2022. Front Pharmacol. 2022. PMID: 35571109 Free PMC article.
-
Regulatory role of local tissue signal Del-1 in cancer and inflammation: a review.Cell Mol Biol Lett. 2021 Jul 3;26(1):31. doi: 10.1186/s11658-021-00274-9. Cell Mol Biol Lett. 2021. PMID: 34217213 Free PMC article. Review.
-
Probiotics and metabolites regulate the oral and gut microbiome composition as host modulation agents in periodontitis: A narrative review.Heliyon. 2023 Feb 3;9(2):e13475. doi: 10.1016/j.heliyon.2023.e13475. eCollection 2023 Feb. Heliyon. 2023. PMID: 36820037 Free PMC article. Review.
-
Identification of key biomarkers in steroid-induced osteonecrosis of the femoral head and their correlation with immune infiltration by bioinformatics analysis.BMC Musculoskelet Disord. 2022 Jan 18;23(1):67. doi: 10.1186/s12891-022-04994-7. BMC Musculoskelet Disord. 2022. PMID: 35042504 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

