Schistosomiasis-from immunopathology to vaccines
- PMID: 32076812
- PMCID: PMC7223304
- DOI: 10.1007/s00281-020-00789-x
Schistosomiasis-from immunopathology to vaccines
Erratum in
-
Correction to: Schistosomiasis-from immunopathology to vaccines.Semin Immunopathol. 2020 Jun;42(3):373-374. doi: 10.1007/s00281-020-00799-9. Semin Immunopathol. 2020. PMID: 32519147 Free PMC article.
Abstract
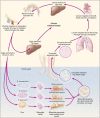
Schistosomiasis (bilharzia) is a neglected tropical disease caused by trematode worms of the genus Schistosoma. The transmission cycle involves human (or other mammalian) water contact with surface water contaminated by faeces or urine, as well as specific freshwater snails acting as intermediate hosts. The main disease-causing species are S. haematobium, S. mansoni and S. japonicum. According to the World Health Organisation, over 250 million people are infected worldwide, leading to considerable morbidity and the estimated loss of 1.9 million disability-adjusted life years (DALYs), a likely underestimated figure. Schistosomiasis is characterised by focal epidemiology and an over-dispersed population distribution, with higher infection rates in children. Complex immune mechanisms lead to the slow acquisition of immune resistance, but innate factors also play a part. Acute schistosomiasis, a feverish syndrome, is most evident in travellers following a primary infection. Chronic schistosomiasis affects mainly individuals with long-standing infections residing in poor rural areas. Immunopathological reactions against schistosome eggs trapped in host tissues lead to inflammatory and obstructive disease in the urinary system (S. haematobium) or intestinal disease, hepatosplenic inflammation and liver fibrosis (S. mansoni and S. japonicum). An effective drug-praziquantel-is available for treatment but, despite intensive efforts, no schistosomiasis vaccines have yet been accepted for public use. In this review, we briefly introduce the schistosome parasites and the immunopathogenic manifestations resulting from schistosomiasis. We then explore aspects of the immunology and host-parasite interplay in schistosome infections paying special attention to the current status of schistosomiasis vaccine development highlighting the advancement of a new controlled human challenge infection model for testing schistosomiasis vaccines.
Keywords: Controlled human infection model; Gastrointestinal/hepatosplenic schistosomiasis; Schistosoma; Schistosomiasis; Schistosomiasis vaccine; Urogenital schistosomiasis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Human schistosomiasis.Lancet. 2006 Sep 23;368(9541):1106-18. doi: 10.1016/S0140-6736(06)69440-3. Lancet. 2006. PMID: 16997665 Review.
-
Efficacy of praziquantel and reinfection patterns in single and mixed infection foci for intestinal and urogenital schistosomiasis in Cameroon.Acta Trop. 2013 Nov;128(2):275-83. doi: 10.1016/j.actatropica.2013.06.007. Epub 2013 Jun 20. Acta Trop. 2013. PMID: 23791803
-
Praziquantel treatment of school children from single and mixed infection foci of intestinal and urogenital schistosomiasis along the Senegal River Basin: monitoring treatment success and re-infection patterns.Acta Trop. 2013 Nov;128(2):292-302. doi: 10.1016/j.actatropica.2012.09.010. Epub 2012 Sep 26. Acta Trop. 2013. PMID: 23022016
-
Schistosomiasis: Drugs used and treatment strategies.Acta Trop. 2017 Dec;176:179-187. doi: 10.1016/j.actatropica.2017.08.002. Epub 2017 Aug 10. Acta Trop. 2017. PMID: 28803725 Review.
-
The impact of single versus mixed schistosome species infections on liver, spleen and bladder morbidity within Malian children pre- and post-praziquantel treatment.BMC Infect Dis. 2010 Jul 29;10:227. doi: 10.1186/1471-2334-10-227. BMC Infect Dis. 2010. PMID: 20670408 Free PMC article.
Cited by
-
Diterpenoids with Schistosomula-Killing and Anti-Fibrosis Activities In Vitro from the Leaves of Croton tiglium.Molecules. 2024 Jan 13;29(2):401. doi: 10.3390/molecules29020401. Molecules. 2024. PMID: 38257314 Free PMC article.
-
Effect of maternal praziquantel treatment for Schistosoma japonicum infection on the offspring susceptibility and immunologic response to infection at age six, a cohort study.PLoS Negl Trop Dis. 2021 Apr 16;15(4):e0009328. doi: 10.1371/journal.pntd.0009328. eCollection 2021 Apr. PLoS Negl Trop Dis. 2021. PMID: 33861768 Free PMC article. Clinical Trial.
-
Schistosoma gallbladder polyp masquerading as a neoplasm: Rare case report and literature review.Clin Case Rep. 2021 Jul 9;9(7):e04420. doi: 10.1002/ccr3.4420. eCollection 2021 Jul. Clin Case Rep. 2021. PMID: 34267906 Free PMC article.
-
Modulation of Host-Parasite Interactions with Small Molecules Targeting Schistosoma mansoni microRNAs.ACS Infect Dis. 2022 Oct 14;8(10):2028-2034. doi: 10.1021/acsinfecdis.2c00360. Epub 2022 Sep 13. ACS Infect Dis. 2022. PMID: 36098656 Free PMC article.
-
Individually or as a Team-The Immunological Milieu in the Lung Caused by Migrating Single-Sex or Mixed-Sex Larvae of Schistosoma mansoni.Pathogens. 2023 Dec 8;12(12):1432. doi: 10.3390/pathogens12121432. Pathogens. 2023. PMID: 38133315 Free PMC article.
References
-
- Boissier J, Grech-Angelini S, Webster BL, Allienne JF, Huyse T, Mas-Coma S, Toulza E, Barré-Cardi H, Rollinson D, Kincaid-Smith J, Oleaga A, Galinier R, Foata J, Rognon A, Berry A, Mouahid G, Henneron R, Moné H, Noel H, Mitta G. Outbreak of urogenital schistosomiasis in Corsica (France): an epidemiological case study. Lancet Infect Dis. 2016;16:971–979. doi: 10.1016/S1473-3099(16)00175-4. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

