Novel Antimicrobial Peptides Formulated in Chitosan Matrices are Effective Against Biofilms of Multidrug-Resistant Wound Pathogens
- PMID: 32074338
- PMCID: PMC7029774
- DOI: 10.1093/milmed/usz222
Novel Antimicrobial Peptides Formulated in Chitosan Matrices are Effective Against Biofilms of Multidrug-Resistant Wound Pathogens
Abstract
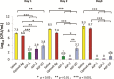
Introduction: Infection frequently complicates the treatment of combat-related wounds, impairs healing, and leads to worse outcomes. To better manage wound infections, antimicrobial therapies that are effective against biofilm and designed for direct wound application are needed. The primary objective of this work was to evaluate a chitosan matrix for delivery of two engineered antimicrobial peptides, (ASP)-1 and ASP-2, to treat biofilm-associated bacteria. A secondary objective was to determine whether replacing the levorotatory (L) form amino acids in ASP-2 with dextrorotatory (D) form amino acids would impact peptide activity.
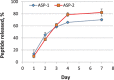
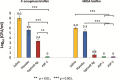
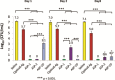
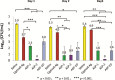
Materials and methods: Chitosan gels loaded with antimicrobial peptides were evaluated for peptide release over 7 days and tested for efficacy against biofilms grown both in vitro on polymer mesh and ex vivo on porcine skin.
Results: When delivered via chitosan, 70% to 80% of peptides were released over 7 days. Gels eradicated biofilms of gram-positive and gram-negative, drug-resistant bacteria in vitro and ex vivo. Under the conditions tested, no meaningful differences in peptide activity between the L and D forms of ASP-2 were detected.
Conclusions: Chitosan serves as an effective delivery platform for ASP-1 and ASP-2 to treat biofilm-embedded bacteria and warrants further development as a topical treatment.
© Association of Military Surgeons of the United States 2020. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
A Novel Peptide-Based Antimicrobial Wound Treatment is Effective Against Biofilms of Multi-Drug Resistant Wound Pathogens.Mil Med. 2018 Mar 1;183(suppl_1):481-486. doi: 10.1093/milmed/usx135. Mil Med. 2018. PMID: 29635548
-
Antibiofilm activity of chitosan/epsilon-poly-L-lysine hydrogels in a porcine ex vivo skin wound polymicrobial biofilm model.Wound Repair Regen. 2021 Mar;29(2):316-326. doi: 10.1111/wrr.12890. Epub 2021 Jan 22. Wound Repair Regen. 2021. PMID: 33480137
-
Nanofiber Dressings Topically Delivering Molecularly Engineered Human Cathelicidin Peptides for the Treatment of Biofilms in Chronic Wounds.Mol Pharm. 2019 May 6;16(5):2011-2020. doi: 10.1021/acs.molpharmaceut.8b01345. Epub 2019 Apr 8. Mol Pharm. 2019. PMID: 30916573
-
Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects.Expert Rev Anti Infect Ther. 2011 Jul;9(7):857-79. doi: 10.1586/eri.11.59. Expert Rev Anti Infect Ther. 2011. PMID: 21810057 Free PMC article. Review.
-
Insights into Peptide Mediated Antibiofilm Treatment in Chronic Wound: A Bench to Bedside Approach.Curr Protein Pept Sci. 2021;22(1):50-59. doi: 10.2174/1389203721666201103084727. Curr Protein Pept Sci. 2021. PMID: 33143623 Review.
Cited by
-
The Expanded Role of Chitosan in Localized Antimicrobial Therapy.Mar Drugs. 2021 Dec 8;19(12):697. doi: 10.3390/md19120697. Mar Drugs. 2021. PMID: 34940696 Free PMC article. Review.
-
Application of Antimicrobial Peptides on Biomedical Implants: Three Ways to Pursue Peptide Coatings.Int J Mol Sci. 2021 Dec 8;22(24):13212. doi: 10.3390/ijms222413212. Int J Mol Sci. 2021. PMID: 34948009 Free PMC article. Review.
-
A Comprehensive Review of Recent Research into the Effects of Antimicrobial Peptides on Biofilms-January 2020 to September 2023.Antibiotics (Basel). 2024 Apr 9;13(4):343. doi: 10.3390/antibiotics13040343. Antibiotics (Basel). 2024. PMID: 38667019 Free PMC article. Review.
-
Anti-Microbial Peptides: Strategies of Design and Development and Their Promising Wound-Healing Activities.Mol Biol Rep. 2022 Sep;49(9):9001-9012. doi: 10.1007/s11033-022-07405-1. Epub 2022 May 8. Mol Biol Rep. 2022. PMID: 35526241 Review.
References
-
- de Veer SJ, Furio L, Harris JM, Hovnanian A: Proteases: common culprits in human skin disorders. Trends Mol Med 2014; 20(3): 166–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

