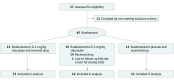
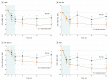
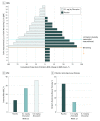
Conflict of Interest Disclosures: Dr Howard has received research support from Alexion Pharmaceuticals, argenx BVBA, the US Centers for Disease Control and Prevention, the Muscular Dystrophy Association (MDA), the National Institutes of Health (NIH; including the National Institute of Neurological Disorders and Stroke and the National Institute of Arthritis and Musculoskeletal and Skin Diseases), argenx and Ra Pharmaceuticals; honoraria/consulting fees from Alexion Pharmaceuticals, argenx, Ra Pharmaceuiticals, and Viela Bio; and nonfinancial support from Alexion Pharmaceuticals, argenx BVBA, Ra Pharmaceuticals, and Toleranzia. Dr Nowak has received research support from the NIH, Genentech, Alexion Pharmaceuticals, Ra Pharmaceuticals, Myasthenia Gravis Foundation of America, Momenta, and Grifols and has served as consultant/advisor for Alexion Pharmaceuticals, CSL Behring, Grifols, Ra Pharmaceuticals, Roivant Sciences, and Momenta. Dr Wolfe is a consultant for Grifols, Takeda, argenx, and Momenta; is on speaker’s bureaus for Grifols and Alexion Pharmaceuiticals; and has received grant/research support from Alexion Pharmaceuticals, argenx, Ra Pharmaceuticals, and Immunovant. Dr Freimer has received honoraria from argenx and Alexion and research support from Catalyst Pharmaceuticals, Ra Pharmaceuticals, Amicus Therapeutics, Alexion Pharmaceuticals, Momenta, Alnylam Pharmaceuticals, UCB, Orphazyme, and NIH (NeuroNExt). Dr Vu serves as site principal investigator for MG clinical trials sponsored by Alexion Pharmaceuticals, argenx, NIH, Grifols, Ra Pharmaceuticals, and UCB and is on speaker’s bureaus for Alexion Pharmaceuticals, Mitsubishi Tanabe Pharma America, CSL Behring, and Allergan. Dr Benatar was site investigator for MG trials sponsored by UCB, Alexion Pharmaceuticals, and an NIH-funded study of rituximab; has reported personal fees from AveXis, Biogen, Genentech, Mitsubishi Tanabe Pharma, and Ra Pharmaceuticals; has provided consulting services to UCB; and has a patent pending for determining the onset of amyotrophic lateral sclerosis. Drs Duda and Farzaneh-Far and Mss Pontius and Hoarty are employees of Ra Pharmaceuticals. Dr Kaminski has received grant 508240 from the MDA; is a consultant for Alnylam Pharmaceuticals, Ra Pharmaceuticals, and UCB; and is CEO and CMO of ARC Biotechnology, LLC, based on US patent 8961981. Dr MacDougall is a consultant for Ra Pharmaceuticals. Dr Barohn served as a consultant for NuFactor and Momenta and receives research support from PTC Therapeutics, Ra Pharmaceuticals, Orphazyme, Sanofi Genzyme, the US Food and Drug Administration (FDA) Office of Orphan Products Development (OOPD), NIH, and Patient-Centered Outcomes Research Institute (PCORI). Dr Dimachkie recently served as a consultant or on speaker’s bureaus for Alnylam, Audentes, CSL Behring, Sanofi Genzyme, Momenta, NuFactor, RMS Medical, Shire Takeda and Terumo and received grants from Alexion Pharmaceuticals, Alnylam Pharmaceuticals, Amicus Therapeutics, Biomarin, Bristol-Myers Squibb, Catalyst, CSL Behring, FDA/OPD, GlaxoSmithKline, Genentech, Grifols, MDA, NIH, Novartis, Genzyme, Octapharma, Orphazyme, UCB, ViroMed Laboratories, and TMA Pharma. Dr Pasnoor has served as a medical advisor or consultant for CSL Behring, Momenta, Alexion Pharmaceuticals, and Terumo BCT. Dr Farmakidis has served as a consultant for Momenta and participated in an advisory board for Terumo BCT. Dr Elsheikh received research support from Biogen, Alexion Pharmaceuticals, and UCB and served as a consultant for Biogen and Bio-Stealth. Dr Gibson has received travel subsidies and honoraria for time spent on advisory boards for Cytokinetics and CSL Behring. Dr Genge is a consultant for Biogen, Sanofi Genzyme, CSL Behring, Mitsubishi Tanabe Pharma America, AL-S Pharma, AB Sciences, Novartis, Wave Life Sciences, argenx, Alexion Pharmaceuticals, AveXis, Akcea Therapeutics, and Cytokinetics. Dr Guidon has been a consultant for Momenta; participated on a medical advisory board with Alexion; has received royalties from Oakstone Publishing; and received clinical trial support from Momenta and PCORI. Dr David has received royalties from Oakstone Publishing. Dr Habib serves on advisory boards for Alexion Pharmaceuticals, and argenx; is on a speaker’s bureau for Alexion Pharmaceuticals; and has received research support from Alexion Pharmaceuticals, Ra Pharmaceuticals, Immunovant, UCB, Acceleron Pharma, and Catalyst Pharmaceuticals. Ms Mathew served as the primary coordinator and evaluator for myasthenia gravis clinical trials sponsored by Alexion Pharmaceuticals, argenx, Grifols, Ra Pharmaceuticals, and UCB and is a consultant for Ra Pharmaceuticals. Dr Mozaffar has received travel subsidies and honoraria for time spent on advisory boards for aTyr Pharma, Alnylam Pharmaceuticals, Alexion Pharmaceuticals, Amicus Therapeutics, argenx, Audentes Therapeutics, Sanofi Genzyme, Sarepta Therapeutics, Spark Therapeutics, Mitsubishi Tanabe Pharma America, and Ultragenyx; served on speaker’s bureaus for Alexion Pharmaceuticals, CSL Behring, Grifols, and Sanofi-Genzyme; received research funding from the Myositis Association, the MDA, the NIH, Alexion Pharmaceuticals, Amicus Therapeutics, argenx, aTyr Pharma, Bristol-Myers Squibb, Idera, Ionis Pharmaceuticals, Grifols, Momenta, Ra Pharmaceuticals, Sanofi-Genzyme, Spark Therapeutics, UCB, Ultragenyx, and Valerion Therapeutics; and serves on the data safety monitoring board for Acceleron and AveXis. Dr Traub has received research support from Alnylam Pharmaceuticals, Akcea Therapeutics, and Vertex Pharmaceuticals and received honoraria/consulting fees from Alnylam Pharmaceuticals, Akcea Therapeutics, and CSL Behring. Dr Chopra has received honoraria from Alexion Pharmaceuticals, and Ra Pharmaceuticals. Dr Khan has participated in advisory boards for Alexion and received a Wellstone trainee clinical fellowship award and NIH research grant 1U54HD08735-01 (2016-2018). Dr Lange has received research support from Cytokinetics, Orion, Orphazyme, Northeast ALS Consortium (NEALS), Lilly/ALS Pharma, ALS Association of Greater New York, Alexion Pharmaceuticals, Acorda Therapeutics, Momenta, Immunovant, UCB, Ra Pharmaceuticals, Baxalta, CSL Behring, and the MDA. Ms Holzberg has received research support from Cytokinetics, Orion, Orphazyme, NEALS, Lilly/ALS Pharma, ALS Association of Greater New York, Alexion Pharmaceuticals, Acorda Therapeutics, Momenta, Immunovant, UCB, Ra Pharmaceuticals, Baxalta, CSL Behring, and the MDA. Dr Khatri has received financial compensation for speaking and research from Teva, Bayer, Biogen, Sanofi Genzyme, Serono EMD, Novartis, Genentech, Acorda, Mallinckrodt, Terumo, and Celgene. Mrs Sershon has received financial compensation for speaking, advisory boards, and consulting from Biogen, Sanofi Genzyme, Serono EMD, Novartis, Genentech, Celgene, and Alexion. Dr Lisak has received research support from the National Multiple Sclerosis Society, the NIH, and Mallinckrodt; participated in clinical trials for Teva, Chugai, MedImmune, Novartis, Genentech (Roche), Alexion Pharmaceuticals, argenx, Ra Pharmaceuticals, and Catalyst Pharmaceuticals; has consulted for and received honoraria from Novartis, argenx, Mallinckrodt, GLG Consulting, Slingshot Consulting, Guidepoint Consulting, Insights Consulting, Schlesinger Group, Clearview Consulting, Alpha Sites Consulting, Putnam Group Consulting, Health Advances, Haven Consulting, Decision Resources Group, and ck2 Medical Communications; has received honoraria from MedDay Pharmaceuticals; has participated in a speaker’s bureau for Teva Pharmaceuticals; and receives publishing royalties from Blackwell Publishers (International Neurology, 2nd edition) and Oxford University Press (Neuroimmunology). Dr Bernitsas has received research support from the NIH, Merck/EMD Serono, Genentech (Roche), Sanofi Genzyme, Mallinckrodt, Chugai, MedImmune, and Novartis and participated in speaker’s bureaus and/or consulted for Celgene, Biogen, Merck/EMD Serono, Bayer, and Sanofi Genzyme. Dr Malik has received research support from Catalyst Pharmaceuticals and PCORI and serves as the director of the MDA-funded clinic at Rush University Medical Center. Ms Lewis-Collins has served as a site clinical research coordinator for Catalyst Pharmaceuticals, PCORI, and Ra Pharmaceuticals. Dr Nicolle has received honoraria from Alexion Pharmaceuticals. Dr Roy has received honoraria from Alexion Pharmaceuticals. Dr Pulley has participated in advisory board meetings for Alexion Pharmaceuticals, Bio Products Laboratory, Stealth BioTherapeutics, CSL Behring, Mitsubishi Tanabe Pharma America, and Catalyst Pharmaceuticals. Dr Berger has received consulting fees for Best Doctors Inc. Dr Sachdev has participated in speaker’s bureaus for CSL Behring, Alexion Pharmaceuticals, Strongbridge Biopharma, and Akcea Therapeutics; has acted as a consultant for argenx and Alexion Pharmaceuticals; has participated as a principal investigator for Catalyst Pharmaceuticals, Alexion Pharmaceuticals, Momenta, Ra Pharmaceuticals, Eisai Corporation, and the MDA; and has been a grant recipient for MDA. Ms Harvey serves as a consultant for Syneos and Ra Pharmaceuticals. Dr Silvestri has served as a consultant for Alexion and argenx, received royalties from Springer Publishing, and has been a speaker for Strongbridge Biopharma. Dr Howard reported grants and nonfinancial support from Ra Pharmaceuticals during the conduct of the study; grants, personal fees, and nonfinancial support from Alexion Pharmaceuticals, and argenx BVBA; and grants from MDA, US Centers for Disease Control and Prevention, and the NIH outside the submitted work. Dr Nowak reported grants and personal fees from Ra Pharmaceuticals during the conduct of the study; grants and personal fees from Alexion Pharmaceuticals, Momenta, Immunovant, argenx; and grants from Annexon Biosciences and Grifols outside the submitted work. Dr Wolfe reported grants from Ra Pharmaceuticals during the conduct of the study; grants from argenx and CSL Behring; and personal fees from Alexion outside the submitted work. Dr Freimer reported personal fees and other support from Ra Pharmaceuticals during the conduct of the study and the submitted work. Dr Vu reported serving as a speaker for Alexion; consulting work for argenx and UCB; and participating in trials in MG sponsored by Alexion Pharmaceuticals, argenx, UCB, and Grifols. Dr Hinton reported grants, personal fees, and nonfinancial support from Ra Pharmaceuticals during the conduct of the study and nonfinancial support from Immunovant outside the submitted work. Dr Benatar reported personal fees from Ra Pharmaceuticals and Viela Bio during the conduct of the study; in addition, Dr Benatar had a patent to Determining Onset of ALS pending. Dr Duda reported other support from Ra Pharmaceuticals during the conduct of the study; in addition, Dr Duda had a patent to listed as an inventor on a patent application relevant to this work pending. Dr MacDougall reported personal fees from Ra Pharmaceuticals during the conduct of the study. Dr Farzaneh-Far is an employee of Ra Pharmaceuticals and holds equity in the company. Dr Kaminski reported personal fees from Ra Pharmaceuticals during the conduct of the study; personal fees from UCB outside the submitted work; and serving as principal investigator for Rare Disease Clinical Research Network for Myasthenia Gravis (MGNet, U54 NS115054). Dr Dimachkie reported grants from University of Kansas during the conduct of the study. Dr Farmakidis reported personal fees from Momenta and Terumo BCT outside the submitted work. Dr Bromberg reported personal fees from Grifols, Alexion Pharmaceuticals, Accordant, and Genzyme outside the submitted work. Dr Elsheikh reported grants and nonfinancial support from Ra Pharmaceuticals during the conduct of the study; grants, personal fees, and nonfinancial support from Biogen; and grants and nonfinancial support from Alexion Pharmaceuticals, UCB, Momenta, and Catalyst Pharmaceuticals outside the submitted work. Dr Genge reported being a site principal investigator for phase 2 study in MG. Dr Guidon reported personal fees and other support from Momenta and other support from Oakstone Publishing and Alexion outside the submitted work. Dr Habib reported grants from Ra Pharmaceuticals during the conduct of the study; grants and other support from Alexion Pharmaceuticals, and argenx; and grants from Immunovant outside the submitted work. Dr Mathew reported personal fees from Ra Pharmaceuticals outside the submitted work. Dr Chopra reported other support from Ra Pharmaceuticals and Alexion Pharmaceuticals, and nonfinancial support from argenx during the conduct of the study. Dr Khan reported personal fees from Alexion and Ra Pharmaceuticals and grants from NIH outside the submitted work. Dr Lange reported grants from Ra Pharmaceuticals during the conduct of the study and from Alexion Pharmaceuticals, Momenta, and Immunovant outside the submitted work. Dr Holzberg reported grants from Ra Pharmaceuticals during the conduct of the study and grants from NIH outside the submitted work. Dr Khatri reported personal fees and nonfinancial support from Biogen, Alexion Pharmaceuticals, Genentech, Sanofi Genzyme, Celgene, and Serono outside the submitted work. Dr Sershon reported personal fees from Alexion Pharmaceuticals, Biogen, Sanofi Genzyme, Novartis, EMD Serono, and Celgene outside the submitted work. Dr Lisak reported grants from Ra Pharmaceuticals during the conduct of the study; grants from Alexion Pharmaceuticals, Genentech (Roche), and MedImmune; grants and personal fees from argenx, Teva, and Catalyst Pharmaceuticals; personal fees from Novartis, Mallinckrodt, GLG Consulting, Alpha Sites, Schelsinger Group, Insights Consulting, Slingshots Consulting, Informa Pharmaceutical Consulting, Health Choices, and Adivo Consulting; and other support from Anavex outside the submitted work. Dr Bernitsas reported grants from Genentech (Roche), Chugai, and Novartis; and personal fees from Biogen and EMD Serono outside the submitted work. Dr Malik reported grants from Ra Pharmaceuticals during the conduct of the study and personal fees from MDA and Conquer MG outside the submitted work. Dr Roy reported grants from Ra Pharmaceuticals during the conduct of the study and personal fees from Alexion Pharmaceuticals, outside the submitted work. Dr Pulley reported personal fees from Alexion Pharmaceuticals, argenx, Bio Products Laboratory, Catalyst Pharmaceuticals, and CSL Behring outside the submitted work. Dr Berger reported grants from University of Florida during the conduct of the study. Dr Shabbir reported grants from Ra Pharmaceuticals during the conduct of the study. Dr Sachdev reported grants from Ra Pharmaceuticals during the conduct of the study; grants and personal fees from Alexion Pharmaceuticals; personal fees from CSL Behring, Strongbridge Biopharma, Akcea Therapeutics, and argenx; and grants from Catalyst Pharmaceuticals, Momenta, Eisai Corporation, and MDA outside the submitted work. Dr Harvey reported personal fees from Alexion Pharmaceuticals, Inc and Ra Pharmaceuticals outside the submitted work. Dr Silvestri reported personal fees from Alexion Pharmaceuticals, and argenx outside the submitted work. Dr Pontius is an employee and holds stock and stock options of Ra Pharmaceuticals outside the submitted work. No other disclosures were reported.