Current advances and outlooks in immunotherapy for pancreatic ductal adenocarcinoma
- PMID: 32061257
- PMCID: PMC7023714
- DOI: 10.1186/s12943-020-01151-3
Current advances and outlooks in immunotherapy for pancreatic ductal adenocarcinoma
Abstract
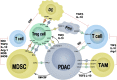
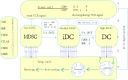
Pancreatic ductal adenocarcinoma (PDAC) is an incurable cancer resistant to traditional treatments, although a limited number of early-stage patients can undergo radical resection. Immunotherapies for the treatment of haematological malignancies as well as solid tumours have been substantially improved over the past decades, and impressive results have been obtained in recent preclinical and clinical trials. However, PDAC is likely the exception because of its unique tumour microenvironment (TME). In this review, we summarize the characteristics of the PDAC TME and focus on the network of various tumour-infiltrating immune cells, outlining the current advances in PDAC immunotherapy and addressing the effect of the PDAC TME on immunotherapy. This review further explores the combinations of different therapies used to enhance antitumour efficacy or reverse immunodeficiencies and describes optimizable immunotherapeutic strategies for PDAC. The concordant combination of various treatments, such as targeting cancer cells and the stroma, reversing suppressive immune reactions and enhancing antitumour reactivity, may be the most promising approach for the treatment of PDAC. Traditional treatments, especially chemotherapy, may also be optimized for individual patients to remodel the immunosuppressive microenvironment for enhanced therapy.
Keywords: Adoptive cell therapy; Immune checkpoint inhibitor; Immunotherapy; Myeloid-derived suppressor cells; Neoantigens; Pancreatic ductal adenocarcinoma; Regulatory T lymphocytes; Tumour microenvironment; Tumour-associated antigens; Tumour-associated macrophages; Tumour-infiltrating lymphocytes; Vaccines.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Targeting both tumour-associated CXCR2+ neutrophils and CCR2+ macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma.Gut. 2018 Jun;67(6):1112-1123. doi: 10.1136/gutjnl-2017-313738. Epub 2017 Dec 1. Gut. 2018. PMID: 29196437 Free PMC article.
-
Immune Checkpoint Inhibition for Pancreatic Ductal Adenocarcinoma: Current Limitations and Future Options.Front Immunol. 2018 Aug 15;9:1878. doi: 10.3389/fimmu.2018.01878. eCollection 2018. Front Immunol. 2018. PMID: 30158932 Free PMC article. Review.
-
Single-cell RNA sequencing reveals compartmental remodeling of tumor-infiltrating immune cells induced by anti-CD47 targeting in pancreatic cancer.J Hematol Oncol. 2019 Nov 27;12(1):124. doi: 10.1186/s13045-019-0822-6. J Hematol Oncol. 2019. PMID: 31771616 Free PMC article.
-
Targeting the tumor microenvironment for pancreatic ductal adenocarcinoma therapy.Chin Clin Oncol. 2019 Apr;8(2):18. doi: 10.21037/cco.2019.03.02. Chin Clin Oncol. 2019. PMID: 31070038 Review.
-
An Immunological Glance on Pancreatic Ductal Adenocarcinoma.Int J Mol Sci. 2020 May 8;21(9):3345. doi: 10.3390/ijms21093345. Int J Mol Sci. 2020. PMID: 32397303 Free PMC article. Review.
Cited by
-
Neuroscience in peripheral cancers: tumors hijacking nerves and neuroimmune crosstalk.MedComm (2020). 2024 Oct 31;5(11):e784. doi: 10.1002/mco2.784. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39492832 Free PMC article. Review.
-
The nine ADAMs family members serve as potential biomarkers for immune infiltration in pancreatic adenocarcinoma.PeerJ. 2020 Sep 30;8:e9736. doi: 10.7717/peerj.9736. eCollection 2020. PeerJ. 2020. PMID: 33062410 Free PMC article.
-
Expression and gene regulatory network of S100A16 protein in cervical cancer cells based on data mining.BMC Cancer. 2023 Nov 17;23(1):1124. doi: 10.1186/s12885-023-11574-y. BMC Cancer. 2023. PMID: 37978469 Free PMC article.
-
Emerging Nanotechnology in Preclinical Pancreatic Cancer Immunotherapy: Driving Towards Clinical Applications.Int J Nanomedicine. 2024 Jul 2;19:6619-6641. doi: 10.2147/IJN.S466459. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38975321 Free PMC article. Review.
-
Construction and validation of a RARRES3-based prognostic signature related to the specific immune microenvironment of pancreatic cancer.Front Oncol. 2024 Feb 5;14:1246308. doi: 10.3389/fonc.2024.1246308. eCollection 2024. Front Oncol. 2024. PMID: 38375157 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

