Clinicopathological Features, Staging, and Current Approaches to Treatment in High-Risk Resectable Melanoma
- PMID: 32061122
- PMCID: PMC7492771
- DOI: 10.1093/jnci/djaa012
Clinicopathological Features, Staging, and Current Approaches to Treatment in High-Risk Resectable Melanoma
Abstract
The incidence of melanoma in the United States has been increasing over the past several decades. Prognosis largely depends on disease stage, with 5-year melanoma-specific survival ranging from as high as 99% in patients with stage I disease to less than 10% for some patients with stage IV (distant metastatic) disease. Fortunately, in the last 5-10 years, there have been remarkable treatment advances for patients with high-risk resectable melanoma, including approval of targeted and immune checkpoint blockade therapies. In addition, results of recent clinical trials have confirmed the importance of sentinel lymph node biopsy and continue to refine the approach to regional lymph node basin management. Lastly, the melanoma staging system was revised in the eighth edition AJCC Cancer Staging Manual, which was implemented on January 1, 2018. Here we discuss these changes and the clinicopathological features that confer high risk for locoregional and distant disease relapse and poor survival. Implications regarding the management of melanoma in the metastatic and adjuvant settings are discussed, as are future directions for neoadjuvant therapies.
© The Author(s) 2020. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
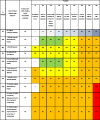

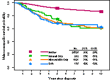
Similar articles
-
Adjuvant therapy of malignant melanoma and the role of sentinel node mapping.Recent Results Cancer Res. 2000;157:178-89. doi: 10.1007/978-3-642-57151-0_15. Recent Results Cancer Res. 2000. PMID: 10857171 Review.
-
Sentinel Lymph Node Biopsy: Past and Present Implications for the Management of Cutaneous Melanoma with Nodal Metastasis.Am J Clin Dermatol. 2018 Nov;19(Suppl 1):24-30. doi: 10.1007/s40257-018-0379-0. Am J Clin Dermatol. 2018. PMID: 30374897 Free PMC article. Review.
-
Recent advances in the care of the patient with malignant melanoma.Ann Surg. 1997 Jan;225(1):1-14. doi: 10.1097/00000658-199701000-00001. Ann Surg. 1997. PMID: 8998115 Free PMC article. Review.
-
Sentinel lymph node biopsy from the vantage point of an oncologic surgeon.Clin Dermatol. 2009 Nov-Dec;27(6):594-6. doi: 10.1016/j.clindermatol.2008.09.017. Clin Dermatol. 2009. PMID: 19880046 Review.
-
Pattern and incidence of first site recurrences following sentinel node procedure in melanoma patients.World J Surg. 2002 Dec;26(12):1405-11. doi: 10.1007/s00268-002-6197-8. Epub 2002 Sep 26. World J Surg. 2002. PMID: 12297910
Cited by
-
The human melanoma proteome atlas-Defining the molecular pathology.Clin Transl Med. 2021 Jul;11(7):e473. doi: 10.1002/ctm2.473. Clin Transl Med. 2021. PMID: 34323403 Free PMC article.
-
Identification of 15 lncRNAs Signature for Predicting Survival Benefit of Advanced Melanoma Patients Treated with Anti-PD-1 Monotherapy.Cells. 2021 Apr 22;10(5):977. doi: 10.3390/cells10050977. Cells. 2021. PMID: 33922038 Free PMC article.
-
Four Immune Modulating Genes in Primary Melanoma That Predict Metastatic Potential.J Surg Res. 2022 Nov;279:682-691. doi: 10.1016/j.jss.2022.06.031. Epub 2022 Aug 5. J Surg Res. 2022. PMID: 35940046 Free PMC article.
-
Identification of fatty acid metabolism-related molecular subtype biomarkers and their correlation with immune checkpoints in cutaneous melanoma.Front Immunol. 2022 Nov 18;13:967277. doi: 10.3389/fimmu.2022.967277. eCollection 2022. Front Immunol. 2022. PMID: 36466837 Free PMC article.
-
Exploration of the immune cell infiltration-related gene signature in the prognosis of melanoma.Aging (Albany NY). 2021 Jan 10;13(3):3459-3482. doi: 10.18632/aging.202279. Epub 2021 Jan 10. Aging (Albany NY). 2021. PMID: 33428606 Free PMC article.
References
-
- Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877–1888. - PubMed
-
- Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386(9992):444–451. - PubMed
-
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30–39. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

