Modified Sialic Acids on Mucus and Erythrocytes Inhibit Influenza A Virus Hemagglutinin and Neuraminidase Functions
- PMID: 32051275
- PMCID: PMC7163148
- DOI: 10.1128/JVI.01567-19
Modified Sialic Acids on Mucus and Erythrocytes Inhibit Influenza A Virus Hemagglutinin and Neuraminidase Functions
Abstract
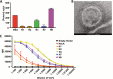
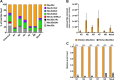
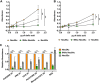
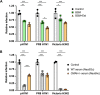
Sialic acids (Sia) are the primary receptors for influenza viruses and are widely displayed on cell surfaces and in secreted mucus. Sia may be present in variant forms that include O-acetyl modifications at C-4, C-7, C-8, and C-9 positions and N-acetyl or N-glycolyl at C-5. They can also vary in their linkages, including α2-3 or α2-6 linkages. Here, we analyze the distribution of modified Sia in cells and tissues of wild-type mice or in mice lacking CMP-N-acetylneuraminic acid hydroxylase (CMAH) enzyme, which synthesizes N-glycolyl (Neu5Gc) modifications. We also examined the variation of Sia forms on erythrocytes and in saliva from different animals. To determine the effect of Sia modifications on influenza A virus (IAV) infection, we tested for effects on hemagglutinin (HA) binding and neuraminidase (NA) cleavage. We confirmed that 9-O-acetyl, 7,9-O-acetyl, 4-O-acetyl, and Neu5Gc modifications are widely but variably expressed in mouse tissues, with the highest levels detected in the respiratory and gastrointestinal (GI) tracts. Secreted mucins in saliva and surface proteins of erythrocytes showed a high degree of variability in display of modified Sia between different species. IAV HAs from different virus strains showed consistently reduced binding to both Neu5Gc- and O-acetyl-modified Sia; however, while IAV NAs were inhibited by Neu5Gc and O-acetyl modifications, there was significant variability between NA types. The modifications of Sia in mucus may therefore have potent effects on the functions of IAV and may affect both pathogens and the normal flora of different mucosal sites.IMPORTANCE Sialic acids (Sia) are involved in numerous different cellular functions and are receptors for many pathogens. Sia come in chemically modified forms, but we lack a clear understanding of how they alter interactions with microbes. Here, we examine the expression of modified Sia in mouse tissues, on secreted mucus in saliva, and on erythrocytes, including those from IAV host species and animals used in IAV research. These Sia forms varied considerably among different animals, and their inhibitory effects on IAV NA and HA activities and on bacterial sialidases (neuraminidases) suggest a host-variable protective role in secreted mucus.
Keywords: influenza; mucus; sialic acids.
Copyright © 2020 American Society for Microbiology.
Figures

Similar articles
-
Expression of 9-O- and 7,9-O-Acetyl Modified Sialic Acid in Cells and Their Effects on Influenza Viruses.mBio. 2019 Dec 3;10(6):e02490-19. doi: 10.1128/mBio.02490-19. mBio. 2019. PMID: 31796537 Free PMC article.
-
Influenza A penetrates host mucus by cleaving sialic acids with neuraminidase.Virol J. 2013 Nov 22;10:321. doi: 10.1186/1743-422X-10-321. Virol J. 2013. PMID: 24261589 Free PMC article.
-
Distribution of O-Acetylated Sialic Acids among Target Host Tissues for Influenza Virus.mSphere. 2017 Sep 6;2(5):e00379-16. doi: 10.1128/mSphere.00379-16. eCollection 2017 Sep-Oct. mSphere. 2017. PMID: 28904995 Free PMC article.
-
Influenza A Virus Hemagglutinin-Neuraminidase-Receptor Balance: Preserving Virus Motility.Trends Microbiol. 2020 Jan;28(1):57-67. doi: 10.1016/j.tim.2019.08.010. Epub 2019 Oct 17. Trends Microbiol. 2020. PMID: 31629602 Free PMC article. Review.
-
[Functional analysis of glyco-molecules that bind with influenza virus].Uirusu. 2016;66(1):101-116. doi: 10.2222/jsv.66.101. Uirusu. 2016. PMID: 28484173 Review. Japanese.
Cited by
-
N-Glycolylneuraminic Acid Binding of Avian and Equine H7 Influenza A Viruses.J Virol. 2022 Mar 9;96(5):e0212021. doi: 10.1128/jvi.02120-21. Epub 2022 Jan 19. J Virol. 2022. PMID: 35044215 Free PMC article.
-
Synthetic Sialosides Terminated with 8-N-Substituted Sialic Acid as Selective Substrates for Sialidases from Bacteria and Influenza Viruses.Angew Chem Int Ed Engl. 2024 Jul 15;63(29):e202403133. doi: 10.1002/anie.202403133. Epub 2024 Jun 14. Angew Chem Int Ed Engl. 2024. PMID: 38713874
-
A mechanism of gene evolution generating mucin function.Sci Adv. 2022 Aug 26;8(34):eabm8757. doi: 10.1126/sciadv.abm8757. Epub 2022 Aug 26. Sci Adv. 2022. PMID: 36026444 Free PMC article.
-
The structure of Phocaeicola vulgatus sialic acid acetylesterase.Acta Crystallogr D Struct Biol. 2022 May 1;78(Pt 5):647-657. doi: 10.1107/S2059798322003357. Epub 2022 Apr 26. Acta Crystallogr D Struct Biol. 2022. PMID: 35503212 Free PMC article.
-
The Nucleoprotein of H7N9 Influenza Virus Positively Regulates TRAF3-Mediated Innate Signaling and Attenuates Viral Virulence in Mice.J Virol. 2020 Nov 23;94(24):e01640-20. doi: 10.1128/JVI.01640-20. Print 2020 Nov 23. J Virol. 2020. PMID: 33028715 Free PMC article.
References
-
- Varki A, Schauer R. 2009. Sialic acids, Chapter 14. In Essentials of glycobiology, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

