Toxoplasma Cathepsin Protease B and Aspartyl Protease 1 Are Dispensable for Endolysosomal Protein Digestion
- PMID: 32051238
- PMCID: PMC7021471
- DOI: 10.1128/mSphere.00869-19
Toxoplasma Cathepsin Protease B and Aspartyl Protease 1 Are Dispensable for Endolysosomal Protein Digestion
Abstract
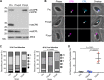
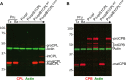
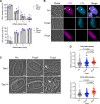
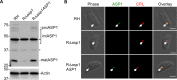
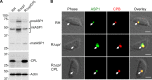
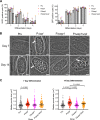
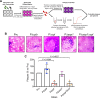
The lysosome-like vacuolar compartment (VAC) is a major site of proteolysis in the intracellular parasite Toxoplasma gondii Previous studies have shown that genetic ablation of a VAC-residing cysteine protease, cathepsin protease L (CPL), resulted in the accumulation of undigested protein in the VAC and loss of parasite viability during the chronic stage of infection. However, since the maturation of another VAC localizing protease, cathepsin protease B (CPB), is dependent on CPL, it remained unknown whether these defects result directly from ablation of CPL or indirectly from a lack of CPB maturation. Likewise, although a previously described cathepsin D-like aspartyl protease 1 (ASP1) could also play a role in proteolysis, its definitive residence and function in the Toxoplasma endolysosomal system were not well defined. Here, we demonstrate that CPB is not necessary for protein turnover in the VAC and that CPB-deficient parasites have normal growth and viability in both the acute and chronic stages of infection. We also show that ASP1 depends on CPL for correct maturation, and it resides in the T. gondii VAC, where, similar to CPB, it plays a dispensable role in protein digestion. Taken together with previous work, our findings suggest that CPL is the dominant protease in a hierarchy of proteolytic enzymes within the VAC. This unusual lack of redundancy for CPL in T. gondii makes it a single exploitable target for disrupting chronic toxoplasmosis.IMPORTANCE Roughly one-third of the human population is chronically infected with the intracellular single-celled parasite Toxoplasma gondii, but little is known about how this organism persists inside people. Previous research suggested that a parasite proteolytic enzyme, termed cathepsin protease L, is important for Toxoplasma persistence; however, it remained possible that other associated proteolytic enzymes could also be involved in the long-term survival of the parasite during infection. Here, we show that two proteolytic enzymes associated with cathepsin protease L play dispensable roles and are dependent on cathepsin L to reach maturity, which differs from the corresponding enzymes in humans. These findings establish a divergent hierarchy of proteases and help focus attention principally on cathepsin protease L as a potential target for interrupting Toxoplasma chronic infection.
Keywords: Toxoplasma gondii; autophagy; cathepsin; lysosome; proteases.
Copyright © 2020 McDonald et al.
Figures

Similar articles
-
A cathepsin C-like protease mediates the post-translation modification of Toxoplasma gondii secretory proteins for optimal invasion and egress.mBio. 2023 Aug 31;14(4):e0017423. doi: 10.1128/mbio.00174-23. Epub 2023 Jun 16. mBio. 2023. PMID: 37326431 Free PMC article.
-
The Toxoplasma plant-like vacuolar compartment (PLVAC).J Eukaryot Microbiol. 2022 Nov;69(6):e12951. doi: 10.1111/jeu.12951. Epub 2022 Oct 27. J Eukaryot Microbiol. 2022. PMID: 36218001 Free PMC article. Review.
-
Role of Toxoplasma gondii Chloroquine Resistance Transporter in Bradyzoite Viability and Digestive Vacuole Maintenance.mBio. 2019 Aug 6;10(4):e01324-19. doi: 10.1128/mBio.01324-19. mBio. 2019. PMID: 31387907 Free PMC article.
-
Non-canonical maturation of two papain-family proteases in Toxoplasma gondii.J Biol Chem. 2013 Feb 1;288(5):3523-34. doi: 10.1074/jbc.M112.443697. Epub 2012 Dec 18. J Biol Chem. 2013. PMID: 23250753 Free PMC article.
-
Cathepsin proteases in Toxoplasma gondii.Adv Exp Med Biol. 2011;712:49-61. doi: 10.1007/978-1-4419-8414-2_4. Adv Exp Med Biol. 2011. PMID: 21660658 Free PMC article. Review.
Cited by
-
Deciphering protein prenylation in endocytic trafficking in Toxoplasma gondii.mBio. 2024 Apr 10;15(4):e0028324. doi: 10.1128/mbio.00283-24. Epub 2024 Feb 26. mBio. 2024. PMID: 38407123 Free PMC article.
-
Toxoplasma TgAtg8-TgAtg3 Interaction Primarily Contributes to Apicoplast Inheritance and Parasite Growth in Tachyzoite.Microbiol Spectr. 2022 Feb 23;10(1):e0149521. doi: 10.1128/spectrum.01495-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196797 Free PMC article.
-
A cathepsin C-like protease mediates the post-translation modification of Toxoplasma gondii secretory proteins for optimal invasion and egress.mBio. 2023 Aug 31;14(4):e0017423. doi: 10.1128/mbio.00174-23. Epub 2023 Jun 16. mBio. 2023. PMID: 37326431 Free PMC article.
-
The Toxoplasma plant-like vacuolar compartment (PLVAC).J Eukaryot Microbiol. 2022 Nov;69(6):e12951. doi: 10.1111/jeu.12951. Epub 2022 Oct 27. J Eukaryot Microbiol. 2022. PMID: 36218001 Free PMC article. Review.
-
Acquisition of Host Cytosolic Protein by Toxoplasma gondii Bradyzoites.mSphere. 2021 Jan 27;6(1):e00934-20. doi: 10.1128/mSphere.00934-20. mSphere. 2021. PMID: 33504659 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

