PTPN22 Acts in a Cell Intrinsic Manner to Restrict the Proliferation and Differentiation of T Cells Following Antibody Lymphodepletion
- PMID: 32047502
- PMCID: PMC6997546
- DOI: 10.3389/fimmu.2020.00052
PTPN22 Acts in a Cell Intrinsic Manner to Restrict the Proliferation and Differentiation of T Cells Following Antibody Lymphodepletion
Abstract
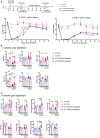
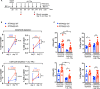
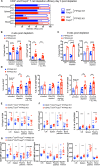
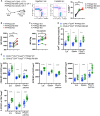
Lymphopenic insult has been shown to precipitate the initiation of autoimmune disease in murine models such as the Non-obese diabetic mouse. Similarly, in man lymphopenia induced by mAb therapy, for instance Alemtuzumab as treatment for Multiple Sclerosis, can precipitate development of secondary autoimmune disease in up to 30 % of patients. We asked whether an identified autoimmune susceptibility locus might increase the risk of developing autoimmunity in the context of mAb-induced lymphopenia in a mouse model. A single nucleotide polymorphism (SNP) in the gene encoding the tyrosine phosphatase PTPN22 (R620W) is associated with multiple human autoimmune diseases, and PTPN22 has been shown to modulate T cell responses, particularly to weak antigens. In keeping with this, PTPN22-deficient or PTPN22 R619W mutant murine T cells adoptively transferred into immunodeficient lymphopenic hosts showed a higher lymphopenia-induced proliferation rate than WT cells. We induced lymphopenia by treating wild-type or PTPN22 knock-out mice with T cell depleting antibodies and monitored reconstitution of the T cell pool. We found that PTPN22 deficient T cells acquired a more activated effector phenotype, with significantly more IFNγ producing cells. This resulted from expansion driven by self-peptide MHC, as it was evident when the contribution of IL-7 to lymphopenic expansion was blocked with IL-7R Ab. Interestingly, Foxp3+ Tregs were also considerably expanded in PTPN22-deficient and PTPN22 R619W mice, as was the frequency of both CD25+ and CD25- CD4 T cells that produce IL-10. Using bone marrow chimeric mice, we showed that PTPN22 influenced development of both regulatory and effector T cell functions in a cell-intrinsic manner. Overall the expansion of Tregs is likely to keep the expanded T effector populations in check and sparing Treg during therapeutic mAb depletion may be a useful strategy to prevent occurrence of secondary autoimmunity.
Keywords: PTPN22; autoimmunity; interleukin-7; lymphopenia; regulatory T cells.
Copyright © 2020 Knipper, Wright, Cope, Malissen and Zamoyska.
Figures

Similar articles
-
Regulation of T Cell Signaling and Immune Responses by PTPN22.Mol Cell Biol. 2024;44(10):443-452. doi: 10.1080/10985549.2024.2378810. Epub 2024 Jul 22. Mol Cell Biol. 2024. PMID: 39039893 Free PMC article. Review.
-
The protein tyrosine phosphatase PTPN22 controls forkhead box protein 3 T regulatory cell induction but is dispensable for T helper type 1 cell polarization.Clin Exp Immunol. 2014 Oct;178(1):178-89. doi: 10.1111/cei.12393. Clin Exp Immunol. 2014. PMID: 24905474 Free PMC article.
-
Depletion of radio-resistant regulatory T cells enhances antitumor immunity during recovery from lymphopenia.Blood. 2012 Sep 20;120(12):2417-27. doi: 10.1182/blood-2012-02-411124. Epub 2012 Jul 17. Blood. 2012. PMID: 22806892
-
Protein tyrosine phosphatase PTPN22 regulates IL-1β dependent Th17 responses by modulating dectin-1 signaling in mice.Eur J Immunol. 2018 Feb;48(2):306-315. doi: 10.1002/eji.201747092. Epub 2017 Oct 20. Eur J Immunol. 2018. PMID: 28948613 Free PMC article.
-
The role of PTPN22 in autoimmunity: learning from mice.Autoimmun Rev. 2014 Mar;13(3):266-71. doi: 10.1016/j.autrev.2013.10.011. Epub 2013 Nov 1. Autoimmun Rev. 2014. PMID: 24189282 Review.
Cited by
-
Regulation of T Cell Signaling and Immune Responses by PTPN22.Mol Cell Biol. 2024;44(10):443-452. doi: 10.1080/10985549.2024.2378810. Epub 2024 Jul 22. Mol Cell Biol. 2024. PMID: 39039893 Free PMC article. Review.
-
Immune Equilibrium Depends on the Interaction Between Recognition and Presentation Landscapes.Front Immunol. 2021 Jul 30;12:706136. doi: 10.3389/fimmu.2021.706136. eCollection 2021. Front Immunol. 2021. PMID: 34394106 Free PMC article. Review.
-
Proautoimmune Allele of Tyrosine Phosphatase, PTPN22, Enhances Tumor Immunity.J Immunol. 2021 Sep 15;207(6):1662-1671. doi: 10.4049/jimmunol.2100304. Epub 2021 Aug 20. J Immunol. 2021. PMID: 34417261 Free PMC article.
-
Modulation of TCR Signaling by Tyrosine Phosphatases: From Autoimmunity to Immunotherapy.Front Cell Dev Biol. 2020 Dec 9;8:608747. doi: 10.3389/fcell.2020.608747. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33425916 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

