Laboratory readiness and response for novel coronavirus (2019-nCoV) in expert laboratories in 30 EU/EEA countries, January 2020
- PMID: 32046815
- PMCID: PMC7029448
- DOI: 10.2807/1560-7917.ES.2020.25.6.2000082
Laboratory readiness and response for novel coronavirus (2019-nCoV) in expert laboratories in 30 EU/EEA countries, January 2020
Abstract
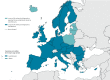
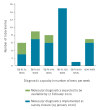
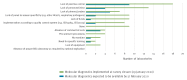
Timely detection of novel coronavirus (2019-nCoV) infection cases is crucial to interrupt the spread of this virus. We assessed the required expertise and capacity for molecular detection of 2019-nCoV in specialised laboratories in 30 European Union/European Economic Area (EU/EEA) countries. Thirty-eight laboratories in 24 EU/EEA countries had diagnostic tests available by 29 January 2020. A coverage of all EU/EEA countries was expected by mid-February. Availability of primers/probes, positive controls and personnel were main implementation barriers.
Keywords: 2019-nCoV; coronavirus; emerging infections; laboratory; response; zoonoses.
Conflict of interest statement
Figures
Similar articles
-
Laboratory capability for molecular detection and confirmation of novel coronavirus in Europe, November 2012.Euro Surveill. 2012 Dec 6;17(49):20335. doi: 10.2807/ese.17.49.20335-en. Euro Surveill. 2012. PMID: 23231892
-
Laboratory capability and surveillance testing for Middle East respiratory syndrome coronavirus infection in the WHO European Region, June 2013.Euro Surveill. 2014 Oct 9;19(40):20923. doi: 10.2807/1560-7917.es2014.19.40.20923. Euro Surveill. 2014. PMID: 25323078
-
Double-quencher probes improve detection sensitivity toward Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in a reverse-transcription polymerase chain reaction (RT-PCR) assay.J Virol Methods. 2020 Oct;284:113926. doi: 10.1016/j.jviromet.2020.113926. Epub 2020 Jul 7. J Virol Methods. 2020. PMID: 32650037 Free PMC article.
-
The Novel Coronavirus: A Bird's Eye View.Int J Occup Environ Med. 2020 Apr;11(2):65-71. doi: 10.15171/ijoem.2020.1921. Epub 2020 Feb 5. Int J Occup Environ Med. 2020. PMID: 32020915 Free PMC article. Review.
-
Challenges in Laboratory Diagnosis of the Novel Coronavirus SARS-CoV-2.Viruses. 2020 May 26;12(6):582. doi: 10.3390/v12060582. Viruses. 2020. PMID: 32466458 Free PMC article. Review.
Cited by
-
Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay.Clin Microbiol Infect. 2020 Jun;26(6):773-779. doi: 10.1016/j.cmi.2020.04.001. Epub 2020 Apr 8. Clin Microbiol Infect. 2020. PMID: 32276116 Free PMC article.
-
SARS-CoV-2/COVID-19: Evidence-Based Recommendations on Diagnosis and Therapy.Geburtshilfe Frauenheilkd. 2020 May;80(5):491-498. doi: 10.1055/a-1156-3991. Epub 2020 May 18. Geburtshilfe Frauenheilkd. 2020. PMID: 32435065 Free PMC article.
-
A Coordinated Public Health Laboratory Response to COVID-19 in Mali.Front Trop Dis. 2021;2:788616. doi: 10.3389/fitd.2021.788616. Epub 2022 Jan 30. Front Trop Dis. 2021. PMID: 39574559 Free PMC article.
-
Unique challenges to control the spread of COVID-19 in the Middle East.J Infect Public Health. 2020 Sep;13(9):1247-1250. doi: 10.1016/j.jiph.2020.06.034. Epub 2020 Jul 13. J Infect Public Health. 2020. PMID: 32690454 Free PMC article.
-
Virus sequencing performance during the SARS-CoV-2 pandemic: a retrospective analysis of data from multiple rounds of external quality assessment in Austria.Front Mol Biosci. 2024 Feb 5;11:1327699. doi: 10.3389/fmolb.2024.1327699. eCollection 2024. Front Mol Biosci. 2024. PMID: 38375507 Free PMC article.
References
-
- Novel 2019 coronavirus genome. [Accessed 02 Feb 2020]. Available from: http://virological.org/t/novel-2019-coronavirus-genome/319
-
- European Centre for Disease Control and Prevention (ECDC). Novel coronavirus. Stockholm: ECDC. [Accessed 11 Feb 2020]. Available from: https://www.ecdc.europa.eu/en/novel-coronavirus-china
-
- World Health Organization (WHO). Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). Geneva: WHO; 30 Jan 2020. [Accessed 01 Feb 2020], Available from: https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

