Magnesium and vitamin C supplementation attenuates steroid-associated osteonecrosis in a rat model
- PMID: 32045781
- PMCID: PMC7185815
- DOI: 10.1016/j.biomaterials.2020.119828
Magnesium and vitamin C supplementation attenuates steroid-associated osteonecrosis in a rat model
Abstract
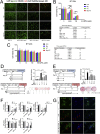
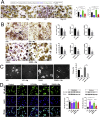
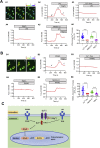
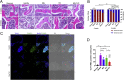
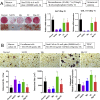
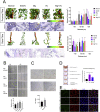
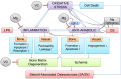
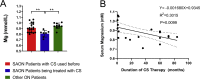
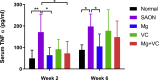
Magnesium (Mg)-based biometal attracts clinical applications due to its biodegradability and beneficial biological effects on tissue regeneration, especially in orthopaedics, yet the underlying anabolic mechanisms in relevant clinical disorders are lacking. The present study investigated the effect of magnesium (Mg) and vitamin C (VC) supplementation for preventing steroid-associated osteonecrosis (SAON) in a rat experimental model. In SAON rats, 50 mg/kg Mg, or 100 mg/kg VC, or combination, or water control was orally supplemented daily for 2 or 6 weeks respectively. Osteonecrosis was evaluated by histology. Serum Mg, VC, and bone turnover markers were measured. Microfil-perfused samples prepared for angiography and trabecular architecture were evaluated by micro-CT. Primary bone marrow cells were isolated from each group to evaluate their potentials in osteoblastogenesis and osteoclastogenesis. The mechanisms were tested in vitro. Histological evaluation showed SAON lesions in steroid treated groups. Mg and VC supplementation synergistically reduced the apoptosis of osteocytes and osteoclast number, and increased osteoblast surface. VC supplementation significantly increased the bone formation marker PINP, and the combination significantly decreased the bone resorption marker CTX. TNFα expression and oxidative injury were decreased in bone marrow in Mg/VC/combination group. Mg significantly increased the blood perfusion in proximal tibia and decreased the leakage particles in distal tibia 2 weeks after SAON induction. VC significantly elevated the osteoblast differentiation potential of marrow cells and improved the trabecular architecture. The combination supplementation significantly inhibited osteoclast differentiation potential of marrow cells. In vitro study showed promoting osteoblast differentiation effect of VC, and anti-inflammation and promoting angiogenesis effect of Mg with underlying mechanisms. Mg and VC supplementation could synergistically alleviate SAON in rats, indicating great translational potentials of metallic minerals for preventing SAON.
Keywords: Corticosteroids; Magnesium; Osteonecrosis; Preclinical studies; Vitamin C.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest All authors declare that they have no conflict of interest.
Figures


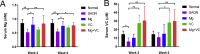

Similar articles
-
Steroid-associated osteonecrosis animal model in rats.J Orthop Translat. 2018 Feb 6;13:13-24. doi: 10.1016/j.jot.2018.01.003. eCollection 2018 Apr. J Orthop Translat. 2018. PMID: 29662787 Free PMC article.
-
A novel bone targeting delivery system carrying phytomolecule icaritin for prevention of steroid-associated osteonecrosis in rats.Bone. 2018 Jan;106:52-60. doi: 10.1016/j.bone.2017.09.011. Epub 2017 Oct 10. Bone. 2018. PMID: 29030232
-
Canonical pathways for validating steroid-associated osteonecrosis in mice.Bone. 2024 Jun;183:117094. doi: 10.1016/j.bone.2024.117094. Epub 2024 Apr 4. Bone. 2024. PMID: 38582289
-
Steroid-associated osteonecrosis: Epidemiology, pathophysiology, animal model, prevention, and potential treatments (an overview).J Orthop Translat. 2015 Jan 13;3(2):58-70. doi: 10.1016/j.jot.2014.12.002. eCollection 2015 Apr. J Orthop Translat. 2015. PMID: 30035041 Free PMC article. Review.
-
Current concepts on the pathogenesis and natural history of steroid-induced osteonecrosis.Clin Rev Allergy Immunol. 2011 Aug;41(1):102-13. doi: 10.1007/s12016-010-8217-z. Clin Rev Allergy Immunol. 2011. PMID: 21161435 Review.
Cited by
-
Laponite intercalated biomimetic multilayer coating prevents glucocorticoids induced orthopedic implant failure.Bioact Mater. 2022 Sep 26;22:60-73. doi: 10.1016/j.bioactmat.2022.09.013. eCollection 2023 Apr. Bioact Mater. 2022. PMID: 36203962 Free PMC article.
-
Modulation of Osteogenesis and Angiogenesis Activities Based on Ionic Release from Zn-Mg Alloys.Materials (Basel). 2022 Oct 13;15(20):7117. doi: 10.3390/ma15207117. Materials (Basel). 2022. PMID: 36295204 Free PMC article.
-
Smart Cargo Delivery System based on Mesoporous Nanoparticles for Bone Disease Diagnosis and Treatment.Adv Sci (Weinh). 2021 Jun;8(12):e2004586. doi: 10.1002/advs.202004586. Epub 2021 Mar 16. Adv Sci (Weinh). 2021. PMID: 34165902 Free PMC article. Review.
-
Local administration of zoledronic acid prevents traumatic osteonecrosis of the femoral head in rat model.J Orthop Translat. 2021 Mar 8;27:132-138. doi: 10.1016/j.jot.2020.08.005. eCollection 2021 Mar. J Orthop Translat. 2021. PMID: 33786320 Free PMC article.
-
Magnesium Picolinate Improves Bone Formation by Regulation of RANK/RANKL/OPG and BMP-2/Runx2 Signaling Pathways in High-Fat Fed Rats.Nutrients. 2021 Sep 24;13(10):3353. doi: 10.3390/nu13103353. Nutrients. 2021. PMID: 34684352 Free PMC article.
References
-
- Zhao D., Witte F., Lu F., Wang J., Li J., Qin L. Current status on clinical applications of magnesium-based orthopaedic implants: a review from clinical translational perspective. Biomaterials. 2017;112:287–302. - PubMed
-
- Tian L., Sheng Y., Huang L., Chow D.H.-K., Chau W.H., Tang N., Ngai T., Wu C., Lu J., Qin L. An innovative Mg/Ti hybrid fixation system developed for fracture fixation and healing enhancement at load-bearing skeletal site. Biomaterials. 2018;180:173–183. - PubMed
-
- Han H.-S., Loffredo S., Jun I., Edwards J., Kim Y.-C., Seok H.-K., Witte F., Mantovani D., Glyn-Jones S. Current status and outlook on the clinical translation of biodegradable metals. Mater. Today. 2019;23:57–71.
-
- Zhao D., Huang S., Lu F., Wang B., Yang L., Qin L., Yang K., Li Y., Li W., Wang W. Vascularized bone grafting fixed by biodegradable magnesium screw for treating osteonecrosis of the femoral head. Biomaterials. 2016;81:84–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

