Lipid Transfer Proteins and Membrane Contact Sites in Human Cancer
- PMID: 32039198
- PMCID: PMC6989408
- DOI: 10.3389/fcell.2019.00371
Lipid Transfer Proteins and Membrane Contact Sites in Human Cancer
Abstract
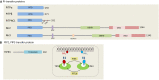
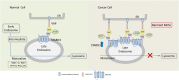
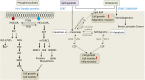
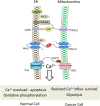
Lipid-transfer proteins (LTPs) were initially discovered as cytosolic factors that facilitate lipid transport between membrane bilayers in vitro. Since then, many LTPs have been isolated from bacteria, plants, yeast, and mammals, and extensively studied in cell-free systems and intact cells. A major advance in the LTP field was associated with the discovery of intracellular membrane contact sites (MCSs), small cytosolic gaps between the endoplasmic reticulum (ER) and other cellular membranes, which accelerate lipid transfer by LTPs. As LTPs modulate the distribution of lipids within cellular membranes, and many lipid species function as second messengers in key signaling pathways that control cell survival, proliferation, and migration, LTPs have been implicated in cancer-associated signal transduction cascades. Increasing evidence suggests that LTPs play an important role in cancer progression and metastasis. This review describes how different LTPs as well as MCSs can contribute to cell transformation and malignant phenotype, and discusses how "aberrant" MCSs are associated with tumorigenesis in human.
Keywords: LTPs; MCSs; calcium; cancer; lipids.
Copyright © 2020 Peretti, Kim, Tufi and Lev.
Figures

Similar articles
-
OSBP-Related Protein Family: Mediators of Lipid Transport and Signaling at Membrane Contact Sites.Int Rev Cell Mol Biol. 2016;321:299-340. doi: 10.1016/bs.ircmb.2015.09.006. Epub 2015 Nov 18. Int Rev Cell Mol Biol. 2016. PMID: 26811291 Review.
-
Ceramide Transport from the Endoplasmic Reticulum to the Trans Golgi Region at Organelle Membrane Contact Sites.Adv Exp Med Biol. 2017;997:69-81. doi: 10.1007/978-981-10-4567-7_5. Adv Exp Med Biol. 2017. PMID: 28815522 Review.
-
The orchestra of lipid-transfer proteins at the crossroads between metabolism and signaling.Prog Lipid Res. 2016 Jan;61:30-9. doi: 10.1016/j.plipres.2015.10.004. Epub 2015 Dec 1. Prog Lipid Res. 2016. PMID: 26658141 Review.
-
Quantitative Models of Lipid Transfer and Membrane Contact Formation.Contact (Thousand Oaks). 2022;5:1-21. doi: 10.1177/25152564221096024. Epub 2022 May 4. Contact (Thousand Oaks). 2022. PMID: 36120532 Free PMC article.
-
Lipid transfer proteins rectify inter-organelle flux and accurately deliver lipids at membrane contact sites.J Lipid Res. 2018 Aug;59(8):1341-1366. doi: 10.1194/jlr.R085324. Epub 2018 Jun 8. J Lipid Res. 2018. PMID: 29884707 Free PMC article. Review.
Cited by
-
Disruption of Mitochondrial-associated ER membranes by HIV-1 tat protein contributes to premature brain aging.CNS Neurosci Ther. 2023 Jan;29(1):365-377. doi: 10.1111/cns.14011. Epub 2022 Nov 23. CNS Neurosci Ther. 2023. PMID: 36419337 Free PMC article.
-
Linking Late Endosomal Cholesterol with Cancer Progression and Anticancer Drug Resistance.Int J Mol Sci. 2022 Jun 29;23(13):7206. doi: 10.3390/ijms23137206. Int J Mol Sci. 2022. PMID: 35806209 Free PMC article. Review.
-
Lipid Transfer Proteins (LTPs)-Structure, Diversity and Roles beyond Antimicrobial Activity.Antibiotics (Basel). 2021 Oct 21;10(11):1281. doi: 10.3390/antibiotics10111281. Antibiotics (Basel). 2021. PMID: 34827219 Free PMC article. Review.
-
A genetic screen to uncover mechanisms underlying lipid transfer protein function at membrane contact sites.Life Sci Alliance. 2024 Mar 18;7(6):e202302525. doi: 10.26508/lsa.202302525. Print 2024 Jun. Life Sci Alliance. 2024. PMID: 38499328 Free PMC article.
-
Role of Lipid Transfer Proteins (LTPs) in the Viral Life Cycle.Front Microbiol. 2021 Jun 23;12:673509. doi: 10.3389/fmicb.2021.673509. eCollection 2021. Front Microbiol. 2021. PMID: 34248884 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

