The stress-activated protein kinase pathway and the expression of stanniocalcin-1 are regulated by miR-146b-5p in papillary thyroid carcinogenesis
- PMID: 32037949
- PMCID: PMC7515490
- DOI: 10.1080/15384047.2020.1721250
The stress-activated protein kinase pathway and the expression of stanniocalcin-1 are regulated by miR-146b-5p in papillary thyroid carcinogenesis
Abstract
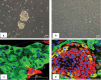
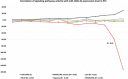
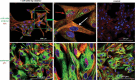
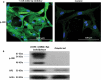
Papillary thyroid cancer (PTC) is the most common type of thyroid cancer. Deciphering the pathophysiological mechanisms that contribute to PTC development is essential to the discovery of optimal diagnostic and therapeutic approaches. MiR-146b-5p has been identified as a cancer-associated microRNA highly up-regulated in PTC. This study explores the hypothesis that miR-146b-5p contributes to papillary thyroid carcinogenesis through regulation of cell signaling pathways in a manner that overcomes the cellular growth suppressive events and provides survival advantage. The effect of miR-146b-5p inhibition on major cancer related signaling pathways and expression of Stanniocalcin-1 (STC1), an emerging molecule associated with stress response and carcinogenesis, was tested in cultured primary thyroid cells using luciferase reporter assays, quantitative real-time PCR, immunofluorescence staining, and flow cytometry. Our results demonstrated that miR-146b-5p inhibits the JNK/AP1 pathway activity and down-regulates the expression of STC-1 in thyroid-cultured cells and in thyroid tissue samples. In the presence of miR-146b-5p, PTC cells were resistant to cell death in response to oxidative stress. This is a novel report that miR-146b-5p directly targets STC1 and regulates the activity of JNK/AP1 pathway. Considering the importance of the JNK/AP1 pathway and STC1 in mediating many physiological and pathological processes like apoptosis, stress response and cellular metabolism, a biological regulator of these pathways would have a great scientific and clinical significance.
Keywords: activator protein-1; miRNA, papillary thyroid carcinoma; stanniocalcin-1; stress-activated protein kinase.
Figures


Similar articles
-
MiR-146b-5p Regulates the Expression of Long Noncoding RNA MALAT1 and Its Effect on the Invasion and Proliferation of Papillary Thyroid Cancer.Cancer Biother Radiopharm. 2021 Jun;36(5):433-440. doi: 10.1089/cbr.2019.3322. Epub 2020 Apr 24. Cancer Biother Radiopharm. 2021. PMID: 32343601
-
Down-regulation of the human major histocompatibility complex class I chain-related gene A (MICA) and its receptor is mediated by microRNA-146b-5p and is a potential mechanism of immunoediting in papillary thyroid carcinoma.Exp Mol Pathol. 2020 Apr;113:104379. doi: 10.1016/j.yexmp.2020.104379. Epub 2020 Jan 11. Exp Mol Pathol. 2020. PMID: 31935378
-
MicroRNA-146b-3p Promotes Cell Metastasis by Directly Targeting NF2 in Human Papillary Thyroid Cancer.Thyroid. 2018 Dec;28(12):1627-1641. doi: 10.1089/thy.2017.0626. Epub 2018 Oct 27. Thyroid. 2018. PMID: 30244634 Free PMC article.
-
MicroRNA-146b: A Novel Biomarker and Therapeutic Target for Human Papillary Thyroid Cancer.Int J Mol Sci. 2017 Mar 15;18(3):636. doi: 10.3390/ijms18030636. Int J Mol Sci. 2017. PMID: 28294980 Free PMC article. Review.
-
Advances on circRNAs Contribute to Carcinogenesis and Progression in Papillary Thyroid Carcinoma.Front Endocrinol (Lausanne). 2021 Jan 21;11:555243. doi: 10.3389/fendo.2020.555243. eCollection 2020. Front Endocrinol (Lausanne). 2021. PMID: 33551989 Free PMC article. Review.
Cited by
-
MiR-146b-5p regulates the scavenging effect of GPx-3 on peroxide in papillary thyroid cancer cells.Heliyon. 2023 Jul 20;9(8):e18489. doi: 10.1016/j.heliyon.2023.e18489. eCollection 2023 Aug. Heliyon. 2023. PMID: 37533981 Free PMC article.
-
Glioblastoma Phagocytic Cell Death: Balancing the Opportunities for Therapeutic Manipulation.Cells. 2024 May 11;13(10):823. doi: 10.3390/cells13100823. Cells. 2024. PMID: 38786045 Free PMC article. Review.
-
PSG7 indicates that age at diagnosis is associated with papillary thyroid carcinoma: A study based on the cancer genome atlas data.Front Genet. 2022 Oct 5;13:952981. doi: 10.3389/fgene.2022.952981. eCollection 2022. Front Genet. 2022. PMID: 36276966 Free PMC article.
-
A new paradigm for epidermal growth factor receptor expression exists in PTC and NIFTP regulated by microRNAs.Front Oncol. 2023 Apr 11;13:1080008. doi: 10.3389/fonc.2023.1080008. eCollection 2023. Front Oncol. 2023. PMID: 37114127 Free PMC article.
References
-
- Vuong HG, Altibi AM, Abdelhamid AH, Ngoc PU, Quan VD, Tantawi MY, Elfil M, Vu TL, Elgebaly A, Oishi N, et al. The changing characteristics and molecular profiles of papillary thyroid carcinoma over time: a systematic review. Oncotarget. 2017;8(6):10637–10649. doi:10.18632/oncotarget.v8i6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
