Urethral dysfunction in a rat model of chemically induced prostatic inflammation: potential involvement of the MRP5 pump
- PMID: 32036697
- PMCID: PMC7099503
- DOI: 10.1152/ajprenal.00566.2019
Urethral dysfunction in a rat model of chemically induced prostatic inflammation: potential involvement of the MRP5 pump
Abstract
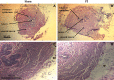
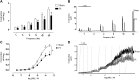
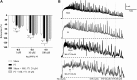
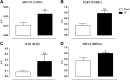
Prostate inflammation (PI) is a clinical condition associated with infection and/or inflammation of the prostate. It is a common disease frequently associated to lower urinary tract (LUT) symptoms. The urethra is an understudied structure in the LUT and plays a fundamental role in the urinary cycle. Here, we proposed to evaluate the effect of PI on the urethra tissue. Male Sprague-Dawley rats were used, and PI was induced by formalin injection into the ventral lobes of the prostate. The pelvic urethra at the prostatic level was harvested for histological analysis, contraction (electrical field stimulation and phenylephrine), and relaxation (sodium nitroprusside/MK-571) experiments. Various gene targets [cytochrome c oxidase subunit 2, transforming growth factor-β1, interleukin-1β, hypoxia-inducible factor-1α, α1A-adrenoceptor, inositol 1,4,5-trisphosphate receptor type 1, voltage-gated Ca2+ channel subunit-α1D, neuronal nitric oxide synthase, soluble guanylyl cyclase, phosphodiesterase 5A, protein kinase CGMP-dependent 1, and multidrug resistance-associated protein 5 (MRP5; ATP-binding cassette subfamily C member 5)] were quantified, and cGMP levels were measured. No histological changes were detected, and functional assays revealed decreased contraction and increased relaxation of urethras from the PI group. The addition of MK-571 to functional assays increased urethral relaxation. Genes associated with inflammation were upregulated in urethras from the PI group, such as cytochrome oxidase c subunit 2, transforming growth factor-β1, interleukin-1β, and hypoxia-inducible factor-1α. We also found increased expression of L-type Ca2+ channels and the neuronal nitric oxide synthase enzyme and decreased expression of the MRP5 pump. Finally, cGMP production was enhanced in urethral tissue of PI animals. The results indicate that PI is associated with proinflammatory gene expression in the urethra without histologically evident inflammation and that PI produces a dysfunctional urethra and MRP5 pump downregulation, which results in cGMP accumulation inside the cell. These findings would help to better understand LUT dysfunctions associated with PI and the role of MRP pumps in the control of LUT function.
Keywords: cGMP; electrical field stimulation; multidrug resistance-associated protein 5; phenylephrine; prostatitis.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures

Similar articles
-
The potential involvement of MRP5 pump in urethral dysfunction in streptozotocin-induced diabetic rats.Int Urol Nephrol. 2023 Feb;55(2):285-293. doi: 10.1007/s11255-022-03405-z. Epub 2022 Nov 3. Int Urol Nephrol. 2023. PMID: 36327005
-
Inhibition of Multidrug Resistance Proteins by MK 571 Enhances Bladder, Prostate, and Urethra Relaxation through cAMP or cGMP Accumulation.J Pharmacol Exp Ther. 2018 Oct;367(1):138-146. doi: 10.1124/jpet.118.250076. Epub 2018 Aug 14. J Pharmacol Exp Ther. 2018. PMID: 30108158
-
Bladder overactivity and afferent hyperexcitability induced by prostate-to-bladder cross-sensitization in rats with prostatic inflammation.J Physiol. 2019 Apr;597(7):2063-2078. doi: 10.1113/JP277452. Epub 2019 Feb 12. J Physiol. 2019. PMID: 30666643 Free PMC article.
-
Cyclic nucleotide metabolism including nitric oxide and phosphodiesterase-related targets in the lower urinary tract.Handb Exp Pharmacol. 2011;(202):527-42. doi: 10.1007/978-3-642-16499-6_23. Handb Exp Pharmacol. 2011. PMID: 21290241 Review.
-
Nitric oxide/cGMP-mediated effects in the outflow region of the lower urinary tract--is there a basis for pharmacological targeting of cGMP?World J Urol. 2005 Dec;23(6):362-7. doi: 10.1007/s00345-005-0019-1. Epub 2005 Nov 8. World J Urol. 2005. PMID: 16283327 Review.
Cited by
-
Time-dependent progress of lower urinary tract dysfunction in streptozotocin-induced diabetic rats with or without low-dose insulin treatment.Int J Med Sci. 2024 Apr 29;21(6):1144-1154. doi: 10.7150/ijms.95461. eCollection 2024. Int J Med Sci. 2024. PMID: 38774757 Free PMC article.
-
Progress and challenges of multidrug resistance proteins in diseases.Am J Cancer Res. 2022 Oct 15;12(10):4483-4501. eCollection 2022. Am J Cancer Res. 2022. PMID: 36381332 Free PMC article. Review.
-
Lipopolysaccharide reduces urethral smooth muscle contractility via cyclooxygenase activation.J Physiol Biochem. 2021 Nov;77(4):557-564. doi: 10.1007/s13105-021-00819-8. Epub 2021 May 21. J Physiol Biochem. 2021. PMID: 34018097
-
Therapeutic effects of a soluble guanylate cyclase activator, BAY 60-2770, on lower urinary tract dysfunction in mice with spinal cord injury.Am J Physiol Renal Physiol. 2022 Oct 1;323(4):F447-F454. doi: 10.1152/ajprenal.00105.2022. Epub 2022 Aug 11. Am J Physiol Renal Physiol. 2022. PMID: 35952343 Free PMC article.
References
-
- Alexandre EC, Calmasini FB, Sponton ACDS, de Oliveira MG, André DM, Silva FH, Delbin MA, Mónica FZ, Antunes E. Influence of the periprostatic adipose tissue in obesity-associated mouse urethral dysfunction and oxidative stress: effect of resveratrol treatment. Eur J Pharmacol 836: 25–33, 2018. doi:10.1016/j.ejphar.2018.08.010. - DOI - PubMed
-
- Alexandre EC, de Oliveira MG, Campos R, Kiguti LR, Calmasini FB, Silva FH, Grant AD, Yoshimura N, Antunes E. How important is the α1-adrenoceptor in primate and rodent proximal urethra? Sex differences in the contribution of α1-adrenoceptor to urethral contractility. Am J Physiol Renal Physiol 312: F1026–F1034, 2017. doi:10.1152/ajprenal.00013.2017. - DOI - PubMed
-
- Alexandre EC, Leiria LO, Silva FH, Mendes-Silvério CB, Calmasini FB, Davel APC, Mónica FZ, De Nucci G, Antunes E. Soluble guanylyl cyclase (sGC) degradation and impairment of nitric oxide-mediated responses in urethra from obese mice: reversal by the sGC activator BAY 60-2770. J Pharmacol Exp Ther 349: 2–9, 2014. doi:10.1124/jpet.113.211029. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

