Immune Responses Raised in an Experimental Colon Carcinoma Model Following Oral Administration of Lactobacillus casei
- PMID: 32033490
- PMCID: PMC7072577
- DOI: 10.3390/cancers12020368
Immune Responses Raised in an Experimental Colon Carcinoma Model Following Oral Administration of Lactobacillus casei
Abstract
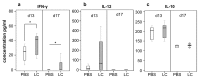
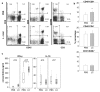
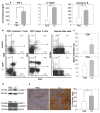
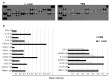
The role of dietary probiotic strains on host anticancer immune responses against experimental colon carcinoma was investigated. We have previously shown that Lactobacillus casei administration led to tumor growth suppression in an experimental colon cancer model. Here, we investigated the underlying immune mechanisms involved in this tumorgrowth inhibitory effect. BALB/c mice received daily live lactobacilli per os prior to the establishment of a syngeneic subcutaneous CT26 tumor. Tumor volume, cytokine production, T cell differentiation and migration, as well as tumor cell apoptosis were examined to outline potential immunomodulatory effects following L. casei oral intake. Probiotic administration in mice resulted in a significant increase in interferon gamma (IFNγ), Granzyme B and chemokine production in the tumor tissue as well as enhanced CD8+ T cell infiltration, accompanied by a suppression of tumor growth. Cytotoxic activity against cancer cells was enhanced in probioticfed compared to control mice, as evidenced by the elevation of apoptotic markers, such as cleaved caspase 3 and poly (ADPribose) polymerase 1 (PARP1), in tumor tissue. Oral administration of Lactobacillus casei induced potent Th1 immune responses and cytotoxic T cell infiltration in the tumor tissue of tumorbearing mice, resulting in tumor growth inhibition. Thus, the microorganism may hold promise as a novel dietary immunoadjuvant in raising protective anticancer immune responses.
Keywords: Lactobacillus casei; immune responses; oral administration; probiotics; syngeneic murine colon carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Lactobacillus casei Exerts Anti-Proliferative Effects Accompanied by Apoptotic Cell Death and Up-Regulation of TRAIL in Colon Carcinoma Cells.PLoS One. 2016 Feb 5;11(2):e0147960. doi: 10.1371/journal.pone.0147960. eCollection 2016. PLoS One. 2016. PMID: 26849051 Free PMC article.
-
Immunomodulatory Effect of Lactobacillus casei in a Murine Model of Colon Carcinogenesis.Probiotics Antimicrob Proteins. 2020 Sep;12(3):1012-1024. doi: 10.1007/s12602-019-09611-z. Probiotics Antimicrob Proteins. 2020. PMID: 31797281
-
Biogenic selenium nanoparticles produced by Lactobacillus casei ATCC 393 inhibit colon cancer cell growth in vitro and in vivo.Nanoscale Adv. 2021 Mar 8;3(9):2516-2528. doi: 10.1039/d0na00984a. eCollection 2021 May 4. Nanoscale Adv. 2021. PMID: 36134160 Free PMC article.
-
Immunomodulatory effects of lactobacillus and Bifidobacterium on both murine and human mitogen-activated T cells.Int Arch Allergy Immunol. 2011;156(2):128-36. doi: 10.1159/000322350. Epub 2011 May 16. Int Arch Allergy Immunol. 2011. PMID: 21576983
-
Modulation of the tumor microenvironment by intratumoral administration of IMO-2125, a novel TLR9 agonist, for cancer immunotherapy.Int J Oncol. 2018 Sep;53(3):1193-1203. doi: 10.3892/ijo.2018.4456. Epub 2018 Jun 27. Int J Oncol. 2018. PMID: 29956749
Cited by
-
From the Colon to the Liver: How Gut Microbiota May Influence Colorectal Cancer Metastatic Potential.J Clin Med. 2024 Jan 12;13(2):420. doi: 10.3390/jcm13020420. J Clin Med. 2024. PMID: 38256554 Free PMC article. Review.
-
Comparison of probiotic Lactobacillus strains isolated from dairy and Iranian traditional food products with those from human source on intestinal microbiota using BALB/C mice model.Braz J Microbiol. 2022 Sep;53(3):1577-1591. doi: 10.1007/s42770-022-00790-6. Epub 2022 Jul 4. Braz J Microbiol. 2022. PMID: 35781865 Free PMC article.
-
Investigation of Immunomodulatory and Gut Microbiota-Altering Properties of Multicomponent Nutraceutical Prepared from Lactic Acid Bacteria, Bovine Colostrum, Apple Production By-Products and Essential Oils.Foods. 2021 Jun 7;10(6):1313. doi: 10.3390/foods10061313. Foods. 2021. PMID: 34200426 Free PMC article.
-
Exploring the Anti-Inflammatory and Antioxidative Potential of Selenium Nanoparticles Biosynthesized by Lactobacillus casei 393 on an Inflamed Caco-2 Cell Line.Cell Biochem Biophys. 2024 Dec;82(4):3265-3276. doi: 10.1007/s12013-024-01356-z. Epub 2024 Sep 11. Cell Biochem Biophys. 2024. PMID: 39261390
-
Mechanism and strategies of immunotherapy resistance in colorectal cancer.Front Immunol. 2022 Sep 27;13:1016646. doi: 10.3389/fimmu.2022.1016646. eCollection 2022. Front Immunol. 2022. PMID: 36238278 Free PMC article. Review.
References
-
- Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

