Contributions of Myosin Light Chain Kinase to Regulation of Epithelial Paracellular Permeability and Mucosal Homeostasis
- PMID: 32028590
- PMCID: PMC7037368
- DOI: 10.3390/ijms21030993
Contributions of Myosin Light Chain Kinase to Regulation of Epithelial Paracellular Permeability and Mucosal Homeostasis
Abstract
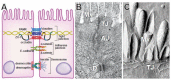
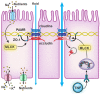
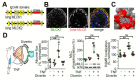
Intestinal barrier function is required for the maintenance of mucosal homeostasis. Barrier dysfunction is thought to promote progression of both intestinal and systemic diseases. In many cases, this barrier loss reflects increased permeability of the paracellular tight junction as a consequence of myosin light chain kinase (MLCK) activation and myosin II regulatory light chain (MLC) phosphorylation. Although some details about MLCK activation remain to be defined, it is clear that this triggers perijunctional actomyosin ring (PAMR) contraction that leads to molecular reorganization of tight junction structure and composition, including occludin endocytosis. In disease states, this process can be triggered by pro-inflammatory cytokines including tumor necrosis factor-α (TNF), interleukin-1β (IL-1β), and several related molecules. Of these, TNF has been studied in the greatest detail and is known to activate long MLCK transcription, expression, enzymatic activity, and recruitment to the PAMR. Unfortunately, toxicities associated with inhibition of MLCK expression or enzymatic activity make these unsuitable as therapeutic targets. Recent work has, however, identified a small molecule that prevents MLCK1 recruitment to the PAMR without inhibiting enzymatic function. This small molecule, termed Divertin, restores barrier function after TNF-induced barrier loss and prevents disease progression in experimental chronic inflammatory bowel disease.
Keywords: ZO-1; actomyosin; barrier function; claudin; cytokines; drug development; inflammatory bowel disease; mucosal immunology; occludin; tight junction.
Conflict of interest statement
W.V.G. is an employee of Thelium Therapeutics, Inc. W.V.G. and J.R.T. are co-founders of Thelium Therapeutics, Inc.
Figures

Similar articles
-
TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis.Gastroenterology. 2013 Aug;145(2):407-15. doi: 10.1053/j.gastro.2013.04.011. Epub 2013 Apr 22. Gastroenterology. 2013. PMID: 23619146 Free PMC article.
-
Intracellular MLCK1 diversion reverses barrier loss to restore mucosal homeostasis.Nat Med. 2019 Apr;25(4):690-700. doi: 10.1038/s41591-019-0393-7. Epub 2019 Apr 1. Nat Med. 2019. PMID: 30936544 Free PMC article.
-
Myosin light chain kinase mediates intestinal barrier dysfunction via occludin endocytosis during anoxia/reoxygenation injury.Am J Physiol Cell Physiol. 2016 Dec 1;311(6):C996-C1004. doi: 10.1152/ajpcell.00113.2016. Epub 2016 Oct 19. Am J Physiol Cell Physiol. 2016. PMID: 27760753
-
The Regulation of Intestinal Mucosal Barrier by Myosin Light Chain Kinase/Rho Kinases.Int J Mol Sci. 2020 May 18;21(10):3550. doi: 10.3390/ijms21103550. Int J Mol Sci. 2020. PMID: 32443411 Free PMC article. Review.
-
IL-1β and the Intestinal Epithelial Tight Junction Barrier.Front Immunol. 2021 Oct 25;12:767456. doi: 10.3389/fimmu.2021.767456. eCollection 2021. Front Immunol. 2021. PMID: 34759934 Free PMC article. Review.
Cited by
-
Intestinal Barrier Dysfunction in Fatty Liver Disease: Roles of Microbiota, Mucosal Immune System, and Bile Acids.Semin Liver Dis. 2022 May;42(2):122-137. doi: 10.1055/s-0042-1748037. Epub 2022 Jun 23. Semin Liver Dis. 2022. PMID: 35738255 Free PMC article.
-
Tea Polyphenols Protects Tracheal Epithelial Tight Junctions in Lung during Actinobacillus pleuropneumoniae Infection via Suppressing TLR-4/MAPK/PKC-MLCK Signaling.Int J Mol Sci. 2023 Jul 24;24(14):11842. doi: 10.3390/ijms241411842. Int J Mol Sci. 2023. PMID: 37511601 Free PMC article.
-
Interaction Between Commensal Bacteria, Immune Response and the Intestinal Barrier in Inflammatory Bowel Disease.Front Immunol. 2021 Nov 11;12:761981. doi: 10.3389/fimmu.2021.761981. eCollection 2021. Front Immunol. 2021. PMID: 34858414 Free PMC article. Review.
-
Cis-Nerolidol Inhibits MAP Kinase and NF-κB Signaling Pathways and Prevents Epithelial Tight Junction Dysfunction in Colon Inflammation: In Vivo and In Vitro Studies.Molecules. 2023 Mar 27;28(7):2982. doi: 10.3390/molecules28072982. Molecules. 2023. PMID: 37049744 Free PMC article.
-
Biochemical Modulators of Tight Junctions (TJs): Occludin, Claudin-2 and Zonulin as Biomarkers of Intestinal Barrier Leakage in the Diagnosis and Assessment of Inflammatory Bowel Disease Progression.Molecules. 2024 Sep 26;29(19):4577. doi: 10.3390/molecules29194577. Molecules. 2024. PMID: 39407507 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

