Extracellular CIRP as an endogenous TREM-1 ligand to fuel inflammation in sepsis
- PMID: 32027618
- PMCID: PMC7141396
- DOI: 10.1172/jci.insight.134172
Extracellular CIRP as an endogenous TREM-1 ligand to fuel inflammation in sepsis
Abstract
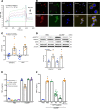
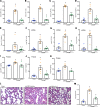
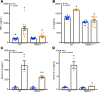
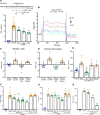
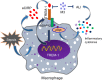
Extracellular cold-inducible RNA-binding protein (eCIRP) is a recently discovered damage-associated molecular pattern. Understanding the precise mechanism by which it exacerbates inflammation is essential. Here we identified that eCIRP is a new biologically active endogenous ligand of triggering receptor expressed on myeloid cells-1 (TREM-1), fueling inflammation in sepsis. Surface plasmon resonance revealed a strong binding affinity between eCIRP and TREM-1, and fluorescence resonance energy transfer assay confirmed eCIRP's interaction with TREM-1 in macrophages. Targeting TREM-1 by its siRNA or a decoy peptide, LP17, or by using TREM-1-/- mice dramatically reduced eCIRP-induced inflammation. We developed a potentially novel 7-aa peptide derived from human eCIRP, M3, which blocked the interaction of TREM-1 and eCIRP. M3 suppressed inflammation induced by eCIRP or agonist TREM-1 antibody cross-linking in murine macrophages or human peripheral blood monocytes. M3 also inhibited eCIRP-induced systemic inflammation and tissue injury. Treatment with M3 further protected mice from sepsis, improved acute lung injury, and increased survival. Thus, we have discovered a potentially novel TREM-1 ligand and developed a new peptide, M3, to block eCIRP-TREM-1 interaction and improve outcomes in sepsis.
Keywords: Bacterial infections; Immunology; Inflammation; Innate immunity; Macrophages.
Conflict of interest statement
Figures

Similar articles
-
Targeting the eCIRP/TREM-1 interaction with a small molecule inhibitor improves cardiac dysfunction in neonatal sepsis.Mol Med. 2020 Dec 4;26(1):121. doi: 10.1186/s10020-020-00243-6. Mol Med. 2020. PMID: 33276725 Free PMC article.
-
BMAL2 promotes eCIRP-induced macrophage endotoxin tolerance.Front Immunol. 2024 Jun 13;15:1426682. doi: 10.3389/fimmu.2024.1426682. eCollection 2024. Front Immunol. 2024. PMID: 38938563 Free PMC article.
-
Inhibition of a triggering receptor expressed on myeloid cells-1 (TREM-1) with an extracellular cold-inducible RNA-binding protein (eCIRP)-derived peptide protects mice from intestinal ischemia-reperfusion injury.Surgery. 2020 Sep;168(3):478-485. doi: 10.1016/j.surg.2020.04.010. Epub 2020 May 18. Surgery. 2020. PMID: 32439208 Free PMC article.
-
Cold-inducible RNA-binding protein (CIRP) in inflammatory diseases: Molecular insights of its associated signalling pathways.Scand J Immunol. 2021 Jan;93(1):e12949. doi: 10.1111/sji.12949. Epub 2020 Sep 8. Scand J Immunol. 2021. PMID: 32738154 Review.
-
Extracellular CIRP (eCIRP) and inflammation.J Leukoc Biol. 2019 Jul;106(1):133-146. doi: 10.1002/JLB.3MIR1118-443R. Epub 2019 Jan 15. J Leukoc Biol. 2019. PMID: 30645013 Free PMC article. Review.
Cited by
-
Synergistic effect of GF9 and streptomycin on relieving gram-negative bacteria-induced sepsis.Front Bioeng Biotechnol. 2022 Aug 30;10:973588. doi: 10.3389/fbioe.2022.973588. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36110326 Free PMC article.
-
DAMPs and radiation injury.Front Immunol. 2024 Jan 25;15:1353990. doi: 10.3389/fimmu.2024.1353990. eCollection 2024. Front Immunol. 2024. PMID: 38333215 Free PMC article. Review.
-
Targeting sting to reduce sepsis-induced acute intestinal injury.Surgery. 2023 Oct;174(4):1071-1077. doi: 10.1016/j.surg.2023.06.032. Epub 2023 Jul 29. Surgery. 2023. PMID: 37517896 Free PMC article.
-
Normothermic machine perfusion attenuates hepatic ischaemia-reperfusion injury by inhibiting CIRP-mediated oxidative stress and mitochondrial fission.J Cell Mol Med. 2021 Dec;25(24):11310-11321. doi: 10.1111/jcmm.17062. Epub 2021 Nov 16. J Cell Mol Med. 2021. PMID: 34786826 Free PMC article.
-
Serum cold-inducible RNA-binding protein (CIRP) levels as a prognostic indicator in patients with acute ischemic stroke.Front Neurol. 2023 Jul 14;14:1211108. doi: 10.3389/fneur.2023.1211108. eCollection 2023. Front Neurol. 2023. PMID: 37521290 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

