PEGylated Liposomal Methyl Prednisolone Succinate does not Induce Infusion Reactions in Patients: A Correlation Between in Vitro Immunological and in Vivo Clinical Studies
- PMID: 32012928
- PMCID: PMC7037198
- DOI: 10.3390/molecules25030558
PEGylated Liposomal Methyl Prednisolone Succinate does not Induce Infusion Reactions in Patients: A Correlation Between in Vitro Immunological and in Vivo Clinical Studies
Abstract
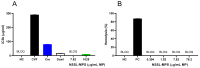
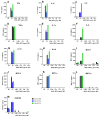
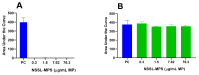
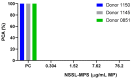
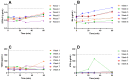
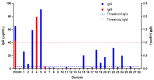
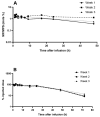
PEGylated nanomedicines are known to induce infusion reactions (IRs) that in some cases can be life-threatening. Herein, we report a case study in which a patient with rare mediastinal and intracardiac IgG4-related sclerosing disease received 8 treatments of intravenously administered PEGylated liposomal methylprednisolone-succinate (NSSL-MPS). Due to the ethical requirements to reduce IRs, the patient received a cocktail of premedication including low dose of steroids, acetaminophen and H2 blockers before each infusion. The treatment was well-tolerated in that IRs, complement activation, anti-PEG antibodies and accelerated blood clearance of the PEGylated drug were not detected. Prior to the clinical study, an in vitro panel of assays utilizing blood of healthy donors was used to determine the potential of a PEGylated drug to activate complement system, elicit pro-inflammatory cytokines, damage erythrocytes and affect various components of the blood coagulation system. The overall findings of the in vitro panel were negative and correlated with the results observed in the clinical phase.
Keywords: IgG4 related disease; PEGylated nanodrugs; anti-PEG antibodies; complement activation; hypersensitive reactions; liposomal steroids.
Conflict of interest statement
Yechezkel Barenholz is an inventor of two patents on NSSL-MPS owned by Yissum TTO of the Hebrew University. Yechezkel Barenholz, Yaacov Naparstek, Yuval Avnir and Rina Ulmansky: “The use of Liposomal Glucocorticoids for Treating Inflammatory States.” US Patent 7,744,920, 2010, June 29, 2010 and Yechezkel Barenholz, Alberto A. Gabizon and Yuval Avnir: “Liposomal Compositions of Glucocorticoid and Glucocorticoid Derivatives”. US Patent 8,932,627, January 13, 2015. These 2 granted patents were not yet licensed.The other authors declare no competing interests.
Figures
Similar articles
-
Understanding the Role of Anti-PEG Antibodies in the Complement Activation by Doxil in Vitro.Molecules. 2018 Jul 12;23(7):1700. doi: 10.3390/molecules23071700. Molecules. 2018. PMID: 30002298 Free PMC article.
-
Pseudo-anaphylaxis to Polyethylene Glycol (PEG)-Coated Liposomes: Roles of Anti-PEG IgM and Complement Activation in a Porcine Model of Human Infusion Reactions.ACS Nano. 2019 Aug 27;13(8):9315-9324. doi: 10.1021/acsnano.9b03942. Epub 2019 Aug 6. ACS Nano. 2019. PMID: 31348638
-
Complement activation by PEGylated liposomes containing prednisolone.Eur J Pharm Sci. 2013 May 13;49(2):265-71. doi: 10.1016/j.ejps.2013.03.007. Epub 2013 Mar 22. Eur J Pharm Sci. 2013. PMID: 23528740
-
The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage.J Control Release. 2013 Nov 28;172(1):38-47. doi: 10.1016/j.jconrel.2013.07.026. Epub 2013 Aug 7. J Control Release. 2013. PMID: 23933235 Review.
-
Activation of complement by therapeutic liposomes and other lipid excipient-based therapeutic products: prediction and prevention.Adv Drug Deliv Rev. 2011 Sep 16;63(12):1020-30. doi: 10.1016/j.addr.2011.06.017. Epub 2011 Jul 14. Adv Drug Deliv Rev. 2011. PMID: 21787819 Review.
Cited by
-
Ultrasound-Guided Microbubble-Mediated Locoregional Delivery of Multiple MicroRNAs Improves Chemotherapy in Hepatocellular Carcinoma.Nanotheranostics. 2022 Jan 1;6(1):62-78. doi: 10.7150/ntno.63320. eCollection 2022. Nanotheranostics. 2022. PMID: 34976581 Free PMC article.
References
-
- Unezaki S., Maruyama K., Hosoda J.-I., Nagae I., Koyanagi Y., Nakata M., Ishida O., Iwatsuru M., Tsuchiya S. Direct measurement of the extravasation of polyethyleneglycol-coated liposomes into solid tumor tissue by in vivo fluorescence microscopy. Int. J. Pharm. 1996;144:11–17. doi: 10.1016/S0378-5173(96)04674-1. - DOI
-
- Anchordoquy T.J., Barenholz Y., Boraschi D., Chorny M., Decuzzi P., Dobrovolskaia M.A., Farhangrazi Z.S., Farrell D., Gabizon A., Ghandehari H., et al. Mechanisms and barriers in cancer nanomedicine: Addressing challenges, looking for solutions. ACS Nano. 2017;11:12–18. doi: 10.1021/acsnano.6b08244. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

