G-protein-coupled estrogen receptor activation upregulates interleukin-1 receptor antagonist in the hippocampus after global cerebral ischemia: implications for neuronal self-defense
- PMID: 32007102
- PMCID: PMC6995076
- DOI: 10.1186/s12974-020-1715-x
G-protein-coupled estrogen receptor activation upregulates interleukin-1 receptor antagonist in the hippocampus after global cerebral ischemia: implications for neuronal self-defense
Abstract
Background: G-protein-coupled estrogen receptor (GPER/GPR30) is a novel membrane-associated estrogen receptor that can induce rapid kinase signaling in various cells. Activation of GPER can prevent hippocampal neuronal cell death following transient global cerebral ischemia (GCI), although the mechanisms remain unclear. In the current study, we sought to address whether GPER activation exerts potent anti-inflammatory effects in the rat hippocampus after GCI as a potential mechanism to limit neuronal cell death.
Methods: GCI was induced by four-vessel occlusion in ovariectomized female SD rats. Specific agonist G1 or antagonist G36 of GPER was administrated using minipump, and antisense oligonucleotide (AS) of interleukin-1β receptor antagonist (IL1RA) was administrated using brain infusion kit. Protein expression of IL1RA, NF-κB-P65, phosphorylation of CREB (p-CREB), Bcl2, cleaved caspase 3, and microglial markers Iba1, CD11b, as well as inflammasome components NLRP3, ASC, cleaved caspase 1, and Cle-IL1β in the hippocampal CA1 region were investigated by immunofluorescent staining and Western blot analysis. The Duolink II in situ proximity ligation assay (PLA) was performed to detect the interaction between NLRP3 and ASC. Immunofluorescent staining for NeuN and TUNEL analysis were used to analyze neuronal survival and apoptosis, respectively. We performed Barnes maze and Novel object tests to compare the cognitive function of the rats.
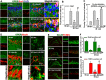
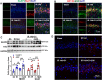
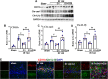
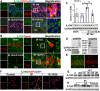
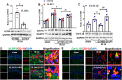
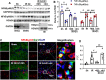
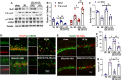
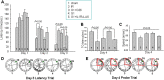
Results: The results showed that G1 attenuated GCI-induced elevation of Iba1 and CD11b in the hippocampal CA1 region at 14 days of reperfusion, and this effect was blocked by G36. G1 treatment also markedly decreased expression of the NLRP3-ASC-caspase 1 inflammasome and IL1β activation, as well as downstream NF-κB signaling, the effects reversed by G36 administration. Intriguingly, G1 caused a robust elevation in neurons of a well-known endogenous anti-inflammatory factor IL1RA, which was reversed by G36 treatment. G1 also enhanced p-CREB level in the hippocampus, a transcription factor known to enhance expression of IL1RA. Finally, in vivo IL1RA-AS abolished the anti-inflammatory, neuroprotective, and anti-apoptotic effects of G1 after GCI and reversed the cognitive-enhancing effects of G1 at 14 days after GCI.
Conclusions: Taken together, the current results suggest that GPER preserves cognitive function following GCI in part by exerting anti-inflammatory effects and enhancing the defense mechanism of neurons by upregulating IL1RA.
Keywords: G-protein-coupled estrogen receptor 1 (GPER/GPR30); Global cerebral ischemia; Inflammasome; Interleukin-1 receptor antagonist (IL1RA); NACHT-, LRR- and PYD-containing protein 3 (NLRP3).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Anti-Inflammatory Actions of G-Protein-Coupled Estrogen Receptor 1 (GPER) and Brain-Derived Estrogen Following Cerebral Ischemia in Ovariectomized Rats.Biology (Basel). 2023 Jan 9;12(1):99. doi: 10.3390/biology12010099. Biology (Basel). 2023. PMID: 36671793 Free PMC article.
-
Intracerebroventricular infusion of secretoneurin inhibits neuronal NLRP3-Apoptosis pathway and preserves learning and memory after cerebral ischemia.Neurochem Int. 2024 Sep;178:105770. doi: 10.1016/j.neuint.2024.105770. Epub 2024 May 16. Neurochem Int. 2024. PMID: 38761854
-
GPR30 mediates estrogen rapid signaling and neuroprotection.Mol Cell Endocrinol. 2014 Apr 25;387(1-2):52-8. doi: 10.1016/j.mce.2014.01.024. Epub 2014 Mar 1. Mol Cell Endocrinol. 2014. PMID: 24594140 Free PMC article.
-
Attenuation of mitochondrial and nuclear p38α signaling: a novel mechanism of estrogen neuroprotection in cerebral ischemia.Mol Cell Endocrinol. 2015 Jan 15;400:21-31. doi: 10.1016/j.mce.2014.11.010. Epub 2014 Nov 25. Mol Cell Endocrinol. 2015. PMID: 25462588
-
Estradiol rescues neurons from global ischemia-induced cell death: multiple cellular pathways of neuroprotection.Steroids. 2009 Jul;74(7):555-61. doi: 10.1016/j.steroids.2009.01.003. Epub 2009 Jan 20. Steroids. 2009. PMID: 19428444 Free PMC article. Review.
Cited by
-
Posttreatment Strategy Against Hypoxia and Ischemia Based on Selective Targeting of Nonnuclear Estrogen Receptors with PaPE-1.Neurotox Res. 2021 Dec;39(6):2029-2041. doi: 10.1007/s12640-021-00441-y. Epub 2021 Nov 19. Neurotox Res. 2021. PMID: 34797527 Free PMC article.
-
Role of estrogen in treatment of female depression.Aging (Albany NY). 2024 Feb 2;16(3):3021-3042. doi: 10.18632/aging.205507. Epub 2024 Feb 2. Aging (Albany NY). 2024. PMID: 38309292 Free PMC article. Review.
-
Alcohol promotes epithelial mesenchymal transformation-mediated premetastatic niche formation of colorectal cancer by activating interaction between laminin-γ2 and integrin-β1.World J Gastroenterol. 2022 Sep 21;28(35):5154-5174. doi: 10.3748/wjg.v28.i35.5154. World J Gastroenterol. 2022. PMID: 36188720 Free PMC article.
-
G protein-coupled estrogen receptor (GPER) in the dorsal hippocampus regulates memory consolidation in gonadectomized male mice, likely via different signaling mechanisms than in female mice.Horm Behav. 2024 May;161:105516. doi: 10.1016/j.yhbeh.2024.105516. Epub 2024 Mar 1. Horm Behav. 2024. PMID: 38428223 Free PMC article.
-
Arsenic Induces Differential Neurotoxicity in Male, Female, and E2-Deficient Females: Comparative Effects on Hippocampal Neurons and Cognition in Adult Rats.Mol Neurobiol. 2022 May;59(5):2729-2744. doi: 10.1007/s12035-022-02770-1. Epub 2022 Feb 17. Mol Neurobiol. 2022. PMID: 35175559
References
-
- Kim YM, Yim HW, Jeong SH, Klem ML, Callaway CW. Does therapeutic hypothermia benefit adult cardiac arrest patients presenting with non-shockable initial rhythms?: a systematic review and meta-analysis of randomized and non-randomized studies. Resuscitation. 2012;83:188–196. doi: 10.1016/j.resuscitation.2011.07.031. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

