Identification of a CD8+ T-cell response to a predicted neoantigen in malignant mesothelioma
- PMID: 32002298
- PMCID: PMC6959430
- DOI: 10.1080/2162402X.2019.1684713
Identification of a CD8+ T-cell response to a predicted neoantigen in malignant mesothelioma
Abstract
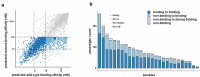
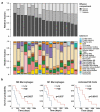
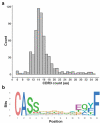
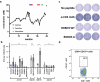
Neoantigens present unique and specific targets for personalized cancer immunotherapy strategies. Given the low mutational burden yet immunotherapy responsiveness of malignant mesothelioma (MM) when compared to other carcinogen-induced malignancies, identifying candidate neoantigens and T cells that recognize them has been a challenge. We used pleural effusions to gain access to MM tumor cells as well as immune cells in order to characterize the tumor-immune interface in MM. We characterized the landscape of potential neoantigens from SNVs identified in 27 MM patients and performed whole transcriptome sequencing of cell populations from 18 patient-matched pleural effusions. IFNγ ELISpot was performed to detect a CD8+ T cell responses to predicted neoantigens in one patient. We detected a median of 68 (range 7-258) predicted neoantigens across the samples. Wild-type non-binding to mutant binding predicted neoantigens increased risk of death in a model adjusting for age, sex, smoking status, histology and treatment (HR: 33.22, CI: 2.55-433.02, p = .007). Gene expression analysis indicated a dynamic immune environment within the pleural effusions. TCR clonotypes increased with predicted neoantigen burden. A strong activated CD8+ T-cell response was identified for a predicted neoantigen produced by a spontaneous mutation in the ROBO3 gene. Despite the challenges associated with the identification of bonafide neoantigens, there is growing evidence that these molecular changes can provide an actionable target for personalized therapeutics in difficult to treat cancers. Our findings support the existence of candidate neoantigens in MM despite the low mutation burden of the tumor, and may present improved treatment opportunities for patients.
Keywords: Malignant mesothelioma; neoantigen; pleural effusion; tumor-immune interface.
© 2019 The Author(s). Published with license by Taylor & Francis Group, LLC.
Figures
Similar articles
-
Immunological ignorance is an enabling feature of the oligo-clonal T cell response to melanoma neoantigens.Proc Natl Acad Sci U S A. 2019 Nov 19;116(47):23662-23670. doi: 10.1073/pnas.1906026116. Epub 2019 Nov 4. Proc Natl Acad Sci U S A. 2019. PMID: 31685621 Free PMC article.
-
The identification of effective tumor-suppressing neoantigens using a tumor-reactive TIL TCR-pMHC ternary complex.Exp Mol Med. 2024 Jun;56(6):1461-1471. doi: 10.1038/s12276-024-01259-2. Epub 2024 Jun 12. Exp Mol Med. 2024. PMID: 38866910 Free PMC article.
-
Utilizing immunogenomic approaches to prioritize targetable neoantigens for personalized cancer immunotherapy.Front Immunol. 2023 Dec 12;14:1301100. doi: 10.3389/fimmu.2023.1301100. eCollection 2023. Front Immunol. 2023. PMID: 38149253 Free PMC article. Review.
-
Recognition of human gastrointestinal cancer neoantigens by circulating PD-1+ lymphocytes.J Clin Invest. 2019 Nov 1;129(11):4992-5004. doi: 10.1172/JCI127967. J Clin Invest. 2019. PMID: 31609250 Free PMC article.
-
The Current Lung Cancer Neoantigen Landscape and Implications for Therapy.J Thorac Oncol. 2021 Jun;16(6):922-932. doi: 10.1016/j.jtho.2021.01.1624. Epub 2021 Feb 10. J Thorac Oncol. 2021. PMID: 33581342 Review.
Cited by
-
SIGANEO: Similarity network with GAN enhancement for immunogenic neoepitope prediction.Comput Struct Biotechnol J. 2023 Oct 31;21:5538-5543. doi: 10.1016/j.csbj.2023.10.050. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 38034402 Free PMC article.
-
Matter of TIME: the tumor-immune microenvironment of mesothelioma and implications for checkpoint blockade efficacy.J Immunother Cancer. 2021 Sep;9(9):e003032. doi: 10.1136/jitc-2021-003032. J Immunother Cancer. 2021. PMID: 34518291 Free PMC article. Review.
-
Integrative transcriptome analysis of malignant pleural mesothelioma reveals a clinically relevant immune-based classification.J Immunother Cancer. 2021 Feb;9(2):e001601. doi: 10.1136/jitc-2020-001601. J Immunother Cancer. 2021. PMID: 33632900 Free PMC article.
-
Stem-like exhausted CD8 T cells in pleural effusions predict improved survival in non-small cell lung cancer (NSCLC) and mesothelioma.Transl Lung Cancer Res. 2024 Sep 30;13(9):2352-2372. doi: 10.21037/tlcr-24-284. Epub 2024 Sep 27. Transl Lung Cancer Res. 2024. PMID: 39430319 Free PMC article.
-
Neoantigen vaccine and neoantigen-specific cell adoptive transfer therapy in solid tumors: Challenges and future directions.Cancer Innov. 2022 Aug 30;1(2):168-182. doi: 10.1002/cai2.26. eCollection 2022 Aug. Cancer Innov. 2022. PMID: 38090649 Free PMC article. Review.
References
-
- Wick DA, Webb JR, Nielsen JS, Martin SD, Kroeger DR, Milne K, Castellarin M, Twumasi-Boateng K, Watson PH, Holt RA, et al. Surveillance of the tumor mutanome by T cells during progression from primary to recurrent ovarian cancer. Clin Cancer Res. 2014;20(5):1125–1134. doi:10.1158/1078-0432.CCR-13-2147. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
