The expression and biological function of chemokine CXCL12 and receptor CXCR4/CXCR7 in placenta accreta spectrum disorders
- PMID: 31991051
- PMCID: PMC7077540
- DOI: 10.1111/jcmm.14990
The expression and biological function of chemokine CXCL12 and receptor CXCR4/CXCR7 in placenta accreta spectrum disorders
Abstract
Objectives: Investigation of mechanism related to excessive invasion of trophoblast cells in placenta accreta spectrum disorders (PAS) provides more strategies and ideas for clinical diagnosis and treatment.
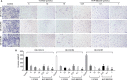
Materials and methods: Blood and placental samples were collected from included patients. The distribution and expression of CXCL12, CXCR4 and CXCR7 proteins in the paraffin of placental tissue in the included cases were analysed, and we analyse the downstream pathways or key proteins involved in cell invasion.
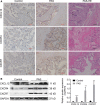
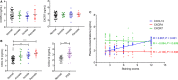
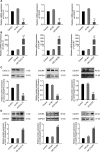
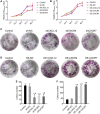
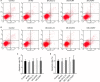
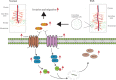
Results: Firstly, our results determined that CXCL12 and CXCR4/CXCR7 were increased in extravillous trophoblastic cell (CXCL12: P < .001; CXCR4: P < .001; CXCR7: P < .001), and the expression levels were closely related to the invasion depth of trophoblastic cells. Secondly, CXCL12 has the potential to become a biochemical indicator of PAS since the high expression of placental trophoblast CXCL12 may be an important source of blood CXCL12. Using lentivirus-mediated RNA interference and overexpression assay, it was found that both chemokine CXCL12 and receptor CXCR4/CXCR7 are associated with regulation of trophoblast cell proliferation, migration and invasion. Further results proved that through the activating the phosphorylation and increasing the expression of MLC and AKT proteins in the Rho/rock, PI3K/AKT signalling pathway, CXCL12, CXCR4 and CXCR7 could up-regulate the expression of RhoA, Rac1 and Cdc42 proteins to promote the migration and invasion of extravillous trophoblastic cell and ultimately formate the placenta accrete compare to the normal placenta.
Conclusions: Our research proved that trophoblasts may contribute to a PAS-associated increase in CXCL12 levels in maternal blood. CXCL12 is not only associated with biological roles of PAS, but may also be potential for prediction of PAS.
Keywords: CXCL12; CXCR4/CXCR7; Function; Invasion; Trophoblast; placenta accreta spectrum disorders.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Androgen receptor and chemokine receptors 4 and 7 form a signaling axis to regulate CXCL12-dependent cellular motility.BMC Cancer. 2015 Mar 31;15:204. doi: 10.1186/s12885-015-1201-5. BMC Cancer. 2015. PMID: 25884570 Free PMC article.
-
Inflammatory CXCL12-CXCR4/CXCR7 axis mediates G-protein signaling pathway to influence the invasion and migration of nasopharyngeal carcinoma cells.Tumour Biol. 2016 Jun;37(6):8169-79. doi: 10.1007/s13277-015-4686-2. Epub 2015 Dec 29. Tumour Biol. 2016. PMID: 26715277
-
Modulatory effects of trophoblast-secreted CXCL12 on the migration and invasion of human first-trimester decidual epithelial cells are mediated by CXCR4 rather than CXCR7.Reprod Biol Endocrinol. 2018 Mar 2;16(1):17. doi: 10.1186/s12958-018-0333-2. Reprod Biol Endocrinol. 2018. PMID: 29499763 Free PMC article.
-
The CXCL12-CXCR4/CXCR7 axis as a mechanism of immune resistance in gastrointestinal malignancies.Semin Cancer Biol. 2020 Oct;65:176-188. doi: 10.1016/j.semcancer.2019.12.007. Epub 2019 Dec 23. Semin Cancer Biol. 2020. PMID: 31874281 Review.
-
Insights into the mechanism of CXCL12-mediated signaling in trophoblast functions and placental angiogenesis.Acta Biochim Biophys Sin (Shanghai). 2015 Sep;47(9):663-72. doi: 10.1093/abbs/gmv064. Epub 2015 Jul 18. Acta Biochim Biophys Sin (Shanghai). 2015. PMID: 26188201 Review.
Cited by
-
Unraveling the molecular mechanisms driving enhanced invasion capability of extravillous trophoblast cells: a comprehensive review.J Assist Reprod Genet. 2024 Mar;41(3):591-608. doi: 10.1007/s10815-024-03036-6. Epub 2024 Feb 5. J Assist Reprod Genet. 2024. PMID: 38315418 Review.
-
CXCL12/CXCR4/CXCR7 axis in placenta tissues of patients with placenta previa.Open Life Sci. 2023 Aug 11;18(1):20220642. doi: 10.1515/biol-2022-0642. eCollection 2023. Open Life Sci. 2023. PMID: 37589008 Free PMC article.
-
Systematic Identification of Hub Genes in Placenta Accreta Spectrum Based on Integrated Transcriptomic and Proteomic Analysis.Front Genet. 2020 Sep 15;11:551495. doi: 10.3389/fgene.2020.551495. eCollection 2020. Front Genet. 2020. PMID: 33101378 Free PMC article.
-
BMP9 maintains the phenotype of HTR-8/Svneo trophoblast cells by activating the SDF1/CXCR4 pathway.BMC Mol Cell Biol. 2023 Aug 7;24(1):24. doi: 10.1186/s12860-023-00487-0. BMC Mol Cell Biol. 2023. PMID: 37550619 Free PMC article.
-
Three categories of similarities between the placenta and cancer that can aid cancer treatment: Cells, the microenvironment, and metabolites.Front Oncol. 2022 Aug 18;12:977618. doi: 10.3389/fonc.2022.977618. eCollection 2022. Front Oncol. 2022. PMID: 36059660 Free PMC article. Review.
References
-
- Tantbirojn P, Crum CP, Parast MM. Pathophysiology of placenta creta: the role of decidua and extravillous trophoblast. Placenta. 2008;29(7):639‐645. - PubMed
-
- Hannon T, Innes BA, Lash GE, Bulmer JN, Robson SC. Effects of local decidua on trophoblast invasion and spiral artery remodeling in focal placenta creta ‐ an immunohistochemical study. Placenta. 2012;33(12):998‐1004. - PubMed
-
- Mcmahon K, Karumanchi SA, Stillman IE, Cummings P, Patton D, Easterling T. Does soluble fms‐like tyrosine kinase‐1 regulate placental invasion? Insight from the invasive placenta. Am J Obstet Gynecol. 2014;210(1):68.e61‐68.e64. - PubMed
-
- Wehrum MJ, Buhimschi IA, Carolyn S, et al. Accreta complicating complete placenta previa is characterized by reduced systemic levels of vascular endothelial growth factor and by epithelial‐to‐mesenchymal transition of the invasive trophoblast. Am J Obstet Gynecol. 2011;204(5):411.e411‐411.e411. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

