Underglycosylated prion protein modulates plaque formation in the brain
- PMID: 31985491
- PMCID: PMC7269559
- DOI: 10.1172/JCI134842
Underglycosylated prion protein modulates plaque formation in the brain
Abstract
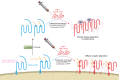
The prion agent is unique in biology and is comprised of prion protein scrapie (PrPSc), a self-templating conformational variant of the host encoded prion protein cellular (PrPC). The deposition patterns of PrPSc in the CNS can vary considerably from a diffuse synaptic pattern to large plaque-like aggregates. Alterations of PrPC posttranslational processing can change PrPSc deposition patterns; however, the mechanism underlying these observations is unclear. In this issue of the JCI, Sevillano and authors determined that parenchymal PrPSc plaques of the mouse brain preferentially incorporated underglycosylated PrPC that had been liberated from the cell surface by the metalloproteinase, ADAM-10, in combination with heparan sulfate. These results provide mechanistic insight into the formation of PrPSc plaques and suggest that PrP posttranslational modifications direct pathogenicity as well as the rate of disease progression.
Conflict of interest statement
Figures
Comment on
-
Prion protein glycans reduce intracerebral fibril formation and spongiosis in prion disease.J Clin Invest. 2020 Mar 2;130(3):1350-1362. doi: 10.1172/JCI131564. J Clin Invest. 2020. PMID: 31985492 Free PMC article.
Similar articles
-
Creutzfeldt-Jakob disease and scrapie prions.Alzheimer Dis Assoc Disord. 1989 Spring-Summer;3(1-2):52-78. doi: 10.1097/00002093-198903010-00007. Alzheimer Dis Assoc Disord. 1989. PMID: 2568118 Review.
-
Genetic and infectious prion diseases.Arch Neurol. 1993 Nov;50(11):1129-53. doi: 10.1001/archneur.1993.00540110011002. Arch Neurol. 1993. PMID: 8105771 Review.
-
Prion encephalopathies of animals and humans.Dev Biol Stand. 1993;80:31-44. Dev Biol Stand. 1993. PMID: 8270114 Review.
-
Immunoaffinity purification and neutralization of scrapie prions.Prog Clin Biol Res. 1989;317:583-600. Prog Clin Biol Res. 1989. PMID: 2574871 Review.
-
Sheep scrapie susceptibility-linked polymorphisms do not modulate the initial binding of cellular to disease-associated prion protein prior to conversion.J Gen Virol. 2005 Sep;86(Pt 9):2627-2634. doi: 10.1099/vir.0.80901-0. J Gen Virol. 2005. PMID: 16099922
Cited by
-
Efficient transmission of human prion diseases to a glycan-free prion protein-expressing host.Brain. 2024 Apr 4;147(4):1539-1552. doi: 10.1093/brain/awad399. Brain. 2024. PMID: 38000783 Free PMC article.
References
-
- Bruce ME, Dickinson AG. Biological stability of different classes of scrapie agent. In: Prusiner SB, Hadlow WJ, eds. Slow transmissible diseases of the nervous system. Vol 2. New York, New York, USA: Academic Press; 1979:71–86.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

