RIP1 kinase activity is critical for skin inflammation but not for viral propagation
- PMID: 31985117
- PMCID: PMC7317411
- DOI: 10.1002/JLB.3MA1219-398R
RIP1 kinase activity is critical for skin inflammation but not for viral propagation
Abstract
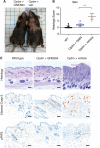
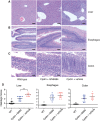
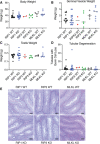
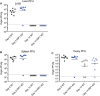
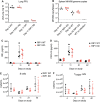
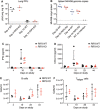
Receptor interacting protein kinase 1 (RIP1) is a critical effector of inflammatory responses and cell death activation. Cell death pathways regulated by RIP1 include caspase-dependent apoptosis and caspase-independent necroptosis. The kinase activity of RIP1 has been associated with a number of inflammatory, neurodegenerative, and oncogenic diseases. In this study, we use the RIP1 kinase inhibitor GNE684 to demonstrate that RIP1 inhibition can effectively block skin inflammation and immune cell infiltrates in livers of Sharpin mutant (Cpdm; chronic proliferative dermatitis) mice in an interventional setting, after disease onset. On the other hand, genetic inactivation of RIP1 (RIP1 KD) or ablation of RIP3 (RIP3 KO) or MLKL (MLKL KO) did not affect testicular pathology of aging male mice. Likewise, infection with vaccinia virus or with mouse gammaherpesvirus MHV68 resulted in similar viral clearance in wild-type, RIP1 KD, and RIP3 KO mice. In summary, this study highlights the benefits of inhibiting RIP1 in skin inflammation, as opposed to its lack of relevance for testicular longevity and the response to certain viral infections.
Keywords: MLKL; RIP1; RIP3; RIPK1; RIPK3; caspase.
© 2020 The Authors. Journal of Leukocyte Biology published by Wiley Periodicals, Inc. on behalf of Society for Leukocyte Biology.
Figures
Similar articles
-
Induction of necroptotic cell death by viral activation of the RIG-I or STING pathway.Cell Death Differ. 2017 Apr;24(4):615-625. doi: 10.1038/cdd.2016.153. Epub 2017 Jan 6. Cell Death Differ. 2017. PMID: 28060376 Free PMC article.
-
Inhibition of receptor-interacting protein kinase 1 improves experimental non-alcoholic fatty liver disease.J Hepatol. 2020 Apr;72(4):627-635. doi: 10.1016/j.jhep.2019.11.008. Epub 2019 Nov 21. J Hepatol. 2020. PMID: 31760070
-
RIP1, RIP3, and MLKL Contribute to Cell Death Caused by Clostridium perfringens Enterotoxin.mBio. 2019 Dec 17;10(6):e02985-19. doi: 10.1128/mBio.02985-19. mBio. 2019. PMID: 31848291 Free PMC article.
-
Necrosis-dependent and independent signaling of the RIP kinases in inflammation.Cytokine Growth Factor Rev. 2014 Apr;25(2):167-74. doi: 10.1016/j.cytogfr.2013.12.013. Epub 2013 Dec 25. Cytokine Growth Factor Rev. 2014. PMID: 24412261 Free PMC article. Review.
-
Necroptosis in health and diseases.Semin Cell Dev Biol. 2014 Nov;35:14-23. doi: 10.1016/j.semcdb.2014.07.013. Epub 2014 Aug 1. Semin Cell Dev Biol. 2014. PMID: 25087983 Review.
Cited by
-
Genetic inactivation of RIP1 kinase activity in rats protects against ischemic brain injury.Cell Death Dis. 2021 Apr 7;12(4):379. doi: 10.1038/s41419-021-03651-6. Cell Death Dis. 2021. PMID: 33828080 Free PMC article.
-
The Role of the Key Effector of Necroptotic Cell Death, MLKL, in Mouse Models of Disease.Biomolecules. 2021 May 28;11(6):803. doi: 10.3390/biom11060803. Biomolecules. 2021. PMID: 34071602 Free PMC article. Review.
-
Cell death pathways: intricate connections and disease implications.EMBO J. 2021 Mar 1;40(5):e106700. doi: 10.15252/embj.2020106700. Epub 2021 Jan 13. EMBO J. 2021. PMID: 33439509 Free PMC article. Review.
-
MLKL deficiency elevates testosterone production in male mice independently of necroptotic functions.Cell Death Dis. 2024 Nov 21;15(11):851. doi: 10.1038/s41419-024-07242-z. Cell Death Dis. 2024. PMID: 39572538 Free PMC article.
-
NF-κB inhibition in keratinocytes causes RIPK1-mediated necroptosis and skin inflammation.Life Sci Alliance. 2021 Apr 15;4(6):e202000956. doi: 10.26508/lsa.202000956. Print 2021 Jun. Life Sci Alliance. 2021. PMID: 33858959 Free PMC article.
References
-
- Newton K. RIPK1 and RIPK3: critical regulators of inflammation and cell death. Trends Cell Biol. 2015;25:347‐353. - PubMed
-
- Silke J, Rickard JA, Gerlic M. The diverse role of RIP kinases in necroptosis and inflammation. Nat Immunol. 2015;16:689‐697. - PubMed
-
- Varfolomeev E, Vucic D. Intracellular regulation of TNF activity in health and disease. Cytokine. 2018;101:26‐32. - PubMed
-
- Vanden Berghe T, Linkermann A, Jouan‐Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non‐apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15:135‐147. - PubMed
-
- Upton JW, Kaiser WJ. DAI Another way: necroptotic control of viral infection. Cell Host Microbe. 2017;21:290‐293. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

