Outpatient parenteral antifungal therapy (OPAT) for invasive fungal infections with intermittent dosing of liposomal amphotericin B
- PMID: 31965178
- PMCID: PMC7527269
- DOI: 10.1093/mmy/myz134
Outpatient parenteral antifungal therapy (OPAT) for invasive fungal infections with intermittent dosing of liposomal amphotericin B
Abstract
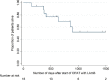
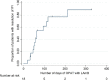
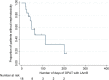
Triazole resistant A. fumigatus has been documented in many parts of the world. In the Netherlands, incidence is now above 10% and results in the need for long-term parenteral therapy with liposomal amphotericin B (LAmB). The long terminal half-life of LAmB suggests that intermittent dosing could be effective, making the application of outpatient antifungal therapy (OPAT) possible. Here, we report our experience with the use of OPAT for Invasive Fungal Infections (IFI). All adult patients treated with LAmB with a 2 or 3 times weekly administration via the outpatient departments in four academic tertiary care centers in the Netherlands and Belgium since January 2010 were included in our analysis. Patient characteristics were collected, as well as information about diagnostics, therapy dose and duration, toxicity, treatment history and outcome of the IFI. In total, 18 patients were included. The most frequently used regimen (67%) was 5 mg/kg 3 times weekly. A partial response to the daily treatment prior to discharge was confirmed by CT-scan in 17 (94%) of patients. A favorable outcome was achieved in 13 (72%) patients. Decrease in renal function occurred in 10 (56%) cases but was reversible in all and was treatment limiting in one patient only. The 100-day mortality and 1-year mortality after initiation of OPAT were 0% and 6%, respectively. In a selected population, and after confirmation of initial response to treatment, our data support the use of OPAT with LAmB for treatment of IFI in an intermittent dosing regimen.
Keywords: Invasive fungal infection; antifungal stewardship; liposomal amphotericin B; outpatient parenteral antibiotic treatment; triazole resistance.
© The Author(s) 2020. Published by Oxford University Press on behalf of The International Society for Human and Animal Mycology.
Figures
Similar articles
-
New pharmacological opportunities for the treatment of invasive mould diseases.J Antimicrob Chemother. 2017 Mar 1;72(suppl_1):i48-i58. doi: 10.1093/jac/dkx033. J Antimicrob Chemother. 2017. PMID: 28355467
-
Tolerability and outcome of once weekly liposomal amphotericin B for the prevention of invasive fungal infections in hematopoietic stem cell transplant patients with graft-versus-host disease.J Oncol Pharm Pract. 2016 Apr;22(2):228-34. doi: 10.1177/1078155214560920. Epub 2014 Dec 3. J Oncol Pharm Pract. 2016. PMID: 25471252 Free PMC article.
-
Treatment of invasive fungal sinusitis with liposomal amphotericin B: a report of four cases.J Med Assoc Thai. 2001 Apr;84(4):593-601. J Med Assoc Thai. 2001. PMID: 11460976
-
Liposomal amphotericin B. Therapeutic use in the management of fungal infections and visceral leishmaniasis.Drugs. 1998 Apr;55(4):585-612. doi: 10.2165/00003495-199855040-00008. Drugs. 1998. PMID: 9561346 Review.
-
Breaking the Mold: A Review of Mucormycosis and Current Pharmacological Treatment Options.Ann Pharmacother. 2016 Sep;50(9):747-57. doi: 10.1177/1060028016655425. Epub 2016 Jun 15. Ann Pharmacother. 2016. PMID: 27307416 Review.
Cited by
-
Current practices and challenges of outpatient parenteral antimicrobial therapy: a narrative review.J Antimicrob Chemother. 2024 Sep 3;79(9):2083-2102. doi: 10.1093/jac/dkae177. J Antimicrob Chemother. 2024. PMID: 38842523 Free PMC article. Review.
-
Critical appraisal beyond clinical guidelines for intraabdominal candidiasis.Crit Care. 2023 Oct 3;27(1):382. doi: 10.1186/s13054-023-04673-6. Crit Care. 2023. PMID: 37789338 Free PMC article. Review.
-
Native valve Aspergillus fumigatus endocarditis in a patient with autoimmune hepatitis on low dose systemic corticosteroids: A case report.IDCases. 2023 Feb 28;31:e01728. doi: 10.1016/j.idcr.2023.e01728. eCollection 2023. IDCases. 2023. PMID: 36911870 Free PMC article.
-
COVID-19 associated mucormycosis: Staging and management recommendations (Report of a multi-disciplinary expert committee).J Oral Biol Craniofac Res. 2021 Oct-Dec;11(4):569-580. doi: 10.1016/j.jobcr.2021.08.001. Epub 2021 Aug 11. J Oral Biol Craniofac Res. 2021. PMID: 34395187 Free PMC article. Review.
-
Successful Use of Multidisciplinary Palliative Care in the Outpatient Treatment of Disseminated Histoplasmosis in an HIV Positive Child.Children (Basel). 2021 Apr 2;8(4):273. doi: 10.3390/children8040273. Children (Basel). 2021. PMID: 33918245 Free PMC article.
References
-
- Girmenia C, Iori AP. An update on the safety and interactions of antifungal drugs in stem cell transplant recipients. Expert Opin Drug Saf. 2017; 16: 329–339. - PubMed
-
- Herbrecht R, Denning DW, Patterson TF et al. .. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002; 347: 408–415. - PubMed
-
- Herbrecht R, Patterson TF, Slavin MA et al. .. Application of the 2008 definitions for invasive fungal diseases to the trial comparing voriconazole versus amphotericin B for therapy of invasive aspergillosis: a collaborative study of the Mycoses Study Group (MSG 05) and the European Organization for Research and Treatment of Cancer Infectious Diseases Group. Clin Infect Dis. 2015; 60: 713–720. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

