A Zeaxanthin-Producing Bacterium Isolated from the Algal Phycosphere Protects Coral Endosymbionts from Environmental Stress
- PMID: 31964724
- PMCID: PMC6974559
- DOI: 10.1128/mBio.01019-19
A Zeaxanthin-Producing Bacterium Isolated from the Algal Phycosphere Protects Coral Endosymbionts from Environmental Stress
Abstract
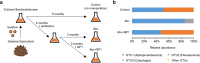
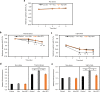
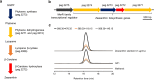
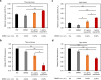
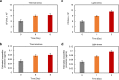
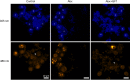
Reef-building corals form a complex consortium with photosynthetic algae in the family Symbiodiniaceae and bacteria, collectively termed the coral holobiont. These bacteria are hypothesized to be involved in the stress resistance of the coral holobiont, but their functional roles remain largely elusive. Here, we show that cultured Symbiodiniaceae algae isolated from the reef-building coral Galaxea fascicularis are associated with novel bacteria affiliated with the family Flavobacteriaceae Antibiotic treatment eliminated the bacteria from cultured Symbiodiniaceae, resulting in a decreased maximum quantum yield of PSII (variable fluorescence divided by maximum fluorescence [Fv/Fm]) and an increased production of reactive oxygen species (ROS) under thermal and light stresses. We then isolated this bacterial strain, named GF1. GF1 inoculation in the antibiotic-treated Symbiodiniaceae cultures restored the Fv/Fm and reduced the ROS production. Furthermore, we found that GF1 produces the carotenoid zeaxanthin, which possesses potent antioxidant activity. Zeaxanthin supplementation to cultured Symbiodiniaceae ameliorated the Fv/Fm and ROS production, suggesting that GF1 mitigates thermal and light stresses in cultured Symbiodiniaceae via zeaxanthin production. These findings could advance our understanding of the roles of bacteria in Symbiodiniaceae and the coral holobiont, thereby contributing to the development of novel approaches toward coral protection through the use of symbiotic bacteria and their metabolites.IMPORTANCE Occupying less than 1% of the seas, coral reefs are estimated to harbor ∼25% of all marine species. However, the destruction of coral reefs has intensified in the face of global climate changes, such as rising seawater temperatures, which induce the overproduction of reactive oxygen species harmful to corals. Although reef-building corals form complex consortia with bacteria and photosynthetic endosymbiotic algae of the family Symbiodiniaceae, the functional roles of coral-associated bacteria remain largely elusive. By manipulating the Symbiodiniaceae bacterial community, we demonstrated that a bacterium that produces an antioxidant carotenoid could mitigate thermal and light stresses in cultured Symbiodiniaceae isolated from a reef-building coral. Therefore, this study illuminates the unexplored roles of coral-associated bacteria under stressful conditions.
Keywords: Symbiodiniaceae; antioxidant; coral; microbiome; stress tolerance; zeaxanthin.
Copyright © 2020 Motone et al.
Figures

Similar articles
-
Mutualistic Interactions between Dinoflagellates and Pigmented Bacteria Mitigate Environmental Stress.Microbiol Spectr. 2023 Feb 14;11(1):e0246422. doi: 10.1128/spectrum.02464-22. Epub 2023 Jan 18. Microbiol Spectr. 2023. PMID: 36651852 Free PMC article.
-
Microbiome community and complexity indicate environmental gradient acclimatisation and potential microbial interaction of endemic coral holobionts in the South China Sea.Sci Total Environ. 2021 Apr 15;765:142690. doi: 10.1016/j.scitotenv.2020.142690. Epub 2020 Oct 3. Sci Total Environ. 2021. PMID: 33071127
-
Symbiodiniaceae-bacteria interactions: rethinking metabolite exchange in reef-building corals as multi-partner metabolic networks.Environ Microbiol. 2020 May;22(5):1675-1687. doi: 10.1111/1462-2920.14918. Epub 2020 Jan 23. Environ Microbiol. 2020. PMID: 31943674 Review.
-
Coral microbiome composition along the northern Red Sea suggests high plasticity of bacterial and specificity of endosymbiotic dinoflagellate communities.Microbiome. 2020 Feb 3;8(1):8. doi: 10.1186/s40168-019-0776-5. Microbiome. 2020. PMID: 32008576 Free PMC article.
-
Coral evolutionary responses to microbial symbioses.Philos Trans R Soc Lond B Biol Sci. 2020 Sep 28;375(1808):20190591. doi: 10.1098/rstb.2019.0591. Epub 2020 Aug 10. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 32772672 Free PMC article. Review.
Cited by
-
Chlamydiae as symbionts of photosynthetic dinoflagellates.ISME J. 2024 Jan 8;18(1):wrae139. doi: 10.1093/ismejo/wrae139. ISME J. 2024. PMID: 39046276 Free PMC article.
-
Long-Term Heat Selection of the Coral Endosymbiont Cladocopium C1acro (Symbiodiniaceae) Stabilizes Associated Bacterial Communities.Int J Mol Sci. 2022 Apr 28;23(9):4913. doi: 10.3390/ijms23094913. Int J Mol Sci. 2022. PMID: 35563303 Free PMC article.
-
The coral microbiome in sickness, in health and in a changing world.Nat Rev Microbiol. 2024 Aug;22(8):460-475. doi: 10.1038/s41579-024-01015-3. Epub 2024 Mar 4. Nat Rev Microbiol. 2024. PMID: 38438489 Review.
-
Description of Prasinibacter corallicola gen. nov., sp. nov., a zeaxanthin-producing bacterium isolated from stony coral Porites lutea.Antonie Van Leeuwenhoek. 2022 Jul;115(7):933-941. doi: 10.1007/s10482-022-01747-3. Epub 2022 May 31. Antonie Van Leeuwenhoek. 2022. PMID: 35639297
-
High Diversity of β-Glucosidase-Producing Bacteria and Their Genes Associated with Scleractinian Corals.Int J Mol Sci. 2021 Mar 29;22(7):3523. doi: 10.3390/ijms22073523. Int J Mol Sci. 2021. PMID: 33805379 Free PMC article.
References
-
- Wilkinson C. (ed). 2004. Status of coral reefs of the world: 2004. Australian Institute of Marine Science, Townsville, Australia.
-
- Moberg F, Folke C. 1999. Ecological goods and services of coral reef ecosystems. Ecol Econ 29:215–233. doi:10.1016/S0921-8009(99)00009-9. - DOI
-
- Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R, Bridge TC, Butler IR, Byrne M, Cantin NE, Comeau S, Connolly SR, Cumming GS, Dalton SJ, Diaz-Pulido G, Eakin CM, Figueira WF, Gilmour JP, Harrison HB, Heron SF, Hoey AS, Hobbs J-PA, Hoogenboom MO, Kennedy EV, Kuo C-Y, Lough JM, Lowe RJ, Liu G, McCulloch MT, Malcolm HA, McWilliam MJ, Pandolfi JM, Pears RJ, Pratchett MS, Schoepf V, Simpson T, Skirving WJ, Sommer B, Torda G, Wachenfeld DR, Willis BL, Wilson SK. 2017. Global warming and recurrent mass bleaching of corals. Nature 543:373–377. doi:10.1038/nature21707. - DOI - PubMed
-
- Carpenter KE, Abrar M, Aeby G, Aronson RB, Banks S, Bruckner A, Chiriboga A, Cortés J, Delbeek JC, Devantier L, Edgar GJ, Edwards AJ, Fenner D, Guzmán HM, Hoeksema BW, Hodgson G, Johan O, Licuanan WY, Livingstone SR, Lovell ER, Moore JA, Obura DO, Ochavillo D, Polidoro BA, Precht WF, Quibilan MC, Reboton C, Richards ZT, Rogers AD, Sanciangco J, Sheppard A, Sheppard C, Smith J, Stuart S, Turak E, Veron JEN, Wallace C, Weil E, Wood E. 2008. One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 321:560–563. doi:10.1126/science.1159196. - DOI - PubMed

