TAK-242, a specific inhibitor of Toll-like receptor 4 signalling, prevents endotoxemia-induced skeletal muscle wasting in mice
- PMID: 31959927
- PMCID: PMC6970997
- DOI: 10.1038/s41598-020-57714-3
TAK-242, a specific inhibitor of Toll-like receptor 4 signalling, prevents endotoxemia-induced skeletal muscle wasting in mice
Abstract
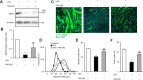
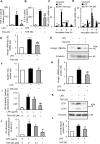
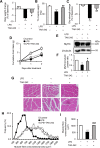
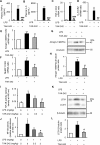
Circulating lipopolysaccharide (LPS) concentrations are often elevated in patients with sepsis or various endogenous diseases related to bacterial translocation from the gut. Systemic inflammatory responses induced by endotoxemia induce severe involuntary loss of skeletal muscle, termed muscle wasting, which adversely affects the survival and functional outcomes of these patients. Currently, no drugs are available for the treatment of endotoxemia-induced skeletal muscle wasting. Here, we tested the effects of TAK-242, a Toll-like receptor 4 (TLR4)-specific signalling inhibitor, on myotube atrophy in vitro and muscle wasting in vivo induced by endotoxin. LPS treatment of murine C2C12 myotubes induced an inflammatory response (increased nuclear factor-κB activity and interleukin-6 and tumour necrosis factor-α expression) and activated the ubiquitin-proteasome and autophagy proteolytic pathways (increased atrogin-1/MAFbx, MuRF1, and LC-II expression), resulting in myotube atrophy. In mice, LPS injection increased the same inflammatory and proteolytic pathways in skeletal muscle and induced atrophy, resulting in reduced grip strength. Notably, pretreatment of cells or mice with TAK-242 reduced or reversed all the detrimental effects of LPS in vitro and in vivo. Collectively, our results indicate that pharmacological inhibition of TLR4 signalling may be a novel therapeutic intervention for endotoxemia-induced muscle wasting.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Lipopolysaccharide inhibits myogenic differentiation of C2C12 myoblasts through the Toll-like receptor 4-nuclear factor-κB signaling pathway and myoblast-derived tumor necrosis factor-α.PLoS One. 2017 Jul 24;12(7):e0182040. doi: 10.1371/journal.pone.0182040. eCollection 2017. PLoS One. 2017. PMID: 28742154 Free PMC article.
-
C188-9, a specific inhibitor of STAT3 signaling, prevents thermal burn-induced skeletal muscle wasting in mice.Front Pharmacol. 2022 Dec 16;13:1031906. doi: 10.3389/fphar.2022.1031906. eCollection 2022. Front Pharmacol. 2022. PMID: 36588738 Free PMC article.
-
Triptolide prevents LPS-induced skeletal muscle atrophy via inhibiting NF-κB/TNF-α and regulating protein synthesis/degradation pathway.Br J Pharmacol. 2021 Aug;178(15):2998-3016. doi: 10.1111/bph.15472. Epub 2021 May 21. Br J Pharmacol. 2021. PMID: 33788266
-
[A Cellular Pharmacological Approach to the Development of Drugs to Treat Muscle Wasting].Yakugaku Zasshi. 2018;138(10):1271-1275. doi: 10.1248/yakushi.18-00091-3. Yakugaku Zasshi. 2018. PMID: 30270271 Review. Japanese.
-
Triggers and mechanisms of skeletal muscle wasting in chronic obstructive pulmonary disease.Int J Biochem Cell Biol. 2013 Oct;45(10):2245-56. doi: 10.1016/j.biocel.2013.06.015. Epub 2013 Jul 1. Int J Biochem Cell Biol. 2013. PMID: 23827718 Review.
Cited by
-
Diverse and multifunctional roles for perlecan (HSPG2) in repair of the intervertebral disc.JOR Spine. 2024 Jul 29;7(3):e1362. doi: 10.1002/jsp2.1362. eCollection 2024 Sep. JOR Spine. 2024. PMID: 39081381 Free PMC article. Review.
-
Biomaterial mediated simultaneous delivery of spermine and alpha ketoglutarate modulate metabolism and innate immune cell phenotype in sepsis mouse models.Biomaterials. 2023 Feb;293:121973. doi: 10.1016/j.biomaterials.2022.121973. Epub 2022 Dec 17. Biomaterials. 2023. PMID: 36549041 Free PMC article.
-
Increased Interleukin-17-Producing γδT Cells in the Brain Exacerbate the Pathogenesis of Sepsis-Associated Encephalopathy and Sepsis-Induced Anxiety in Mice.J Clin Med. 2023 Jun 27;12(13):4309. doi: 10.3390/jcm12134309. J Clin Med. 2023. PMID: 37445343 Free PMC article.
-
Human platelets display dysregulated sepsis-associated autophagy, induced by altered LC3 protein-protein interaction of the Vici-protein EPG5.Autophagy. 2022 Jul;18(7):1534-1550. doi: 10.1080/15548627.2021.1990669. Epub 2021 Nov 18. Autophagy. 2022. PMID: 34689707 Free PMC article.
-
Toll-Like Receptors as a Therapeutic Target in the Era of Immunotherapies.Front Cell Dev Biol. 2021 Oct 4;9:756315. doi: 10.3389/fcell.2021.756315. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34671606 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

