The kinesin-5 tail domain directly modulates the mechanochemical cycle of the motor domain for anti-parallel microtubule sliding
- PMID: 31958056
- PMCID: PMC7015671
- DOI: 10.7554/eLife.51131
The kinesin-5 tail domain directly modulates the mechanochemical cycle of the motor domain for anti-parallel microtubule sliding
Abstract
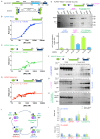
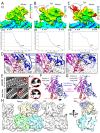
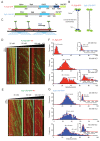
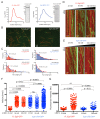
Kinesin-5 motors organize mitotic spindles by sliding apart microtubules. They are homotetramers with dimeric motor and tail domains at both ends of a bipolar minifilament. Here, we describe a regulatory mechanism involving direct binding between tail and motor domains and its fundamental role in microtubule sliding. Kinesin-5 tails decrease microtubule-stimulated ATP-hydrolysis by specifically engaging motor domains in the nucleotide-free or ADP states. Cryo-EM reveals that tail binding stabilizes an open motor domain ATP-active site. Full-length motors undergo slow motility and cluster together along microtubules, while tail-deleted motors exhibit rapid motility without clustering. The tail is critical for motors to zipper together two microtubules by generating substantial sliding forces. The tail is essential for mitotic spindle localization, which becomes severely reduced in tail-deleted motors. Our studies suggest a revised microtubule-sliding model, in which kinesin-5 tails stabilize motor domains in the microtubule-bound state by slowing ATP-binding, resulting in high-force production at both homotetramer ends.
Keywords: D. melanogaster; Microtubule; cell biology; human; kinesin-5; mitosis; mitotic spindle; molecular biophysics; motor protein; sliding; structural biology.
© 2020, Bodrug et al.
Conflict of interest statement
TB, EW, SN, AT, AA, IG, JM, GD, SI, PG, LG, RM, CS, RM, JS, SR, SF, JA No competing interests declared
Figures



Similar articles
-
Conserved mechanisms of microtubule-stimulated ADP release, ATP binding, and force generation in transport kinesins.Elife. 2014 Sep 10;3:e03680. doi: 10.7554/eLife.03680. Elife. 2014. PMID: 25209998 Free PMC article.
-
Kinesin-5/Cut7 C-terminal tail phosphorylation is essential for microtubule sliding force and bipolar mitotic spindle assembly.Curr Biol. 2024 Oct 21;34(20):4781-4793.e6. doi: 10.1016/j.cub.2024.08.035. Epub 2024 Oct 15. Curr Biol. 2024. PMID: 39413787
-
The kinesin-5 tail and bipolar minifilament domains are the origin of its microtubule crosslinking and sliding activity.Mol Biol Cell. 2023 Oct 1;34(11):ar111. doi: 10.1091/mbc.E23-07-0287. Epub 2023 Aug 23. Mol Biol Cell. 2023. PMID: 37610838 Free PMC article.
-
New insights into the mechanochemical coupling mechanism of kinesin-microtubule complexes from their high-resolution structures.Biochem Soc Trans. 2023 Aug 31;51(4):1505-1520. doi: 10.1042/BST20221238. Biochem Soc Trans. 2023. PMID: 37560910 Free PMC article. Review.
-
Mechanisms by Which Kinesin-5 Motors Perform Their Multiple Intracellular Functions.Int J Mol Sci. 2021 Jun 15;22(12):6420. doi: 10.3390/ijms22126420. Int J Mol Sci. 2021. PMID: 34203964 Free PMC article. Review.
Cited by
-
Effect of Kinesin-5 Tail Domain on Motor Dynamics for Antiparallel Microtubule Sliding.Int J Mol Sci. 2021 Jul 23;22(15):7857. doi: 10.3390/ijms22157857. Int J Mol Sci. 2021. PMID: 34360622 Free PMC article.
-
Effects of microtubule length and crowding on active microtubule network organization.iScience. 2023 Jan 27;26(2):106063. doi: 10.1016/j.isci.2023.106063. eCollection 2023 Feb 17. iScience. 2023. PMID: 36852161 Free PMC article.
-
Mitotic Functions and Characters of KIF11 in Cancers.Biomolecules. 2024 Mar 22;14(4):386. doi: 10.3390/biom14040386. Biomolecules. 2024. PMID: 38672404 Free PMC article. Review.
-
Cortical dynein drives centrosome clustering in cells with centrosome amplification.Mol Biol Cell. 2023 May 15;34(6):ar63. doi: 10.1091/mbc.E22-07-0296. Epub 2023 Apr 5. Mol Biol Cell. 2023. PMID: 37017483 Free PMC article.
-
Mechanism and regulation of kinesin motors.Nat Rev Mol Cell Biol. 2024 Oct 11. doi: 10.1038/s41580-024-00780-6. Online ahead of print. Nat Rev Mol Cell Biol. 2024. PMID: 39394463 Review.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
- R01 NS073610/NS/NINDS NIH HHS/United States
- R01 GM110530/GM/NIGMS NIH HHS/United States
- R01 GM110283/GM/NIGMS NIH HHS/United States
- 1615991/National Science Foundation
- BSF-2015851/United States-Israel Binational Science Foundation
- ISF 386/18/Israel Science Foundation
- R01 GM052468/GM/NIGMS NIH HHS/United States
- R37 GM052468/GM/NIGMS NIH HHS/United States
- R01 GM130556/GM/NIGMS NIH HHS/United States
- R01 NS118513/NS/NINDS NIH HHS/United States
- P50 CA097247/CA/NCI NIH HHS/United States
- R01 GM121491/GM/NIGMS NIH HHS/United States
- R35 GM124889/GM/NIGMS NIH HHS/United States

