Reduced Influence of apoE on Aβ43 Aggregation and Reduced Vascular Aβ43 Toxicity as Compared with Aβ40 and Aβ42
- PMID: 31953617
- PMCID: PMC7118029
- DOI: 10.1007/s12035-020-01873-x
Reduced Influence of apoE on Aβ43 Aggregation and Reduced Vascular Aβ43 Toxicity as Compared with Aβ40 and Aβ42
Abstract
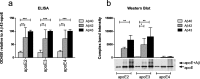
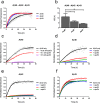
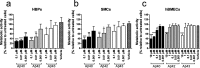
The amyloid-β 43 (Aβ43) peptide has been shown to be abundantly expressed in Alzheimer's disease plaques, whereas only relatively low levels have been demonstrated in cerebral amyloid angiopathy (CAA). To better understand this discrepant distribution, we studied various biochemical properties of Aβ43, in comparison with Aβ40 and Aβ42. We assessed the interaction of Aβ43 with the three apoE isoforms (apoE2, apoE3, and apoE4) using SDS-PAGE/Western blotting and ELISA, aggregation propensity using thioflavin T assays, and cytotoxicity towards cerebrovascular cells using MTT assays. We found that Aβ43 did not differ from Aβ42 in its interaction with apoE, whereas Aβ40 had a significantly lower degree of interaction with apoE. At a molar ratio of 1:100 (apoE:Aβ), all apoE isoforms were comparably capable of inhibiting aggregation of Aβ40 and Aβ42, but not Aβ43. All Aβ variants had a concentration-dependent negative effect on metabolic activity of cerebrovascular cells. However, the degree of this effect differed for the three Aβ isoforms (Aβ40 > Aβ42 > Aβ43), with Aβ43 being the least cytotoxic peptide towards cerebrovascular cells. We conclude that Aβ43 has different biochemical characteristics compared with Aβ40 and Aβ42. Aggregation of Aβ43 is not inhibited by apoE, in contrast to the aggregation of Aβ40 and Aβ42. Furthermore, cerebrovascular cells are less sensitive towards Aβ43, compared with Aβ40 and Aβ42. In contrast, Aβ43 neither differed from Aβ42 in its aggregation propensity (in the absence of apoE) nor in its apoE-binding capacity. Altogether, our findings may provide an explanation for the lower levels of Aβ43 accumulation in cerebral vessel walls.
Keywords: Aggregation; Alzheimer’s disease; Amyloid-β (1–43); Apolipoprotein E; Cerebral amyloid angiopathy; Cytotoxicity.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Distinctive contribution of two additional residues in protein aggregation of Aβ42 and Aβ40 isoforms.BMB Rep. 2024 Jun;57(6):263-272. doi: 10.5483/BMBRep.2024-0044. BMB Rep. 2024. PMID: 38835114 Free PMC article. Review.
-
Different Aβ43 deposition patterns in the brains of aged dogs, sea lions, and cats.J Vet Med Sci. 2022 Nov 18;84(12):1563-1573. doi: 10.1292/jvms.22-0386. Epub 2022 Oct 25. J Vet Med Sci. 2022. PMID: 36288928 Free PMC article.
-
Aβ43 in human Alzheimer's disease: effects of active Aβ42 immunization.Acta Neuropathol Commun. 2019 Sep 2;7(1):141. doi: 10.1186/s40478-019-0791-6. Acta Neuropathol Commun. 2019. PMID: 31477180 Free PMC article.
-
Comparison of neurotoxicity of different aggregated forms of Aβ40, Aβ42 and Aβ43 in cell cultures.J Pept Sci. 2017 Mar;23(3):245-251. doi: 10.1002/psc.2975. Epub 2017 Feb 16. J Pept Sci. 2017. PMID: 28211253
-
The interaction of amyloid-beta with ApoE.Subcell Biochem. 2005;38:255-72. doi: 10.1007/0-387-23226-5_13. Subcell Biochem. 2005. PMID: 15709483 Review.
Cited by
-
Oligomer Formation by Physiologically Relevant C-Terminal Isoforms of Amyloid β-Protein.Biomolecules. 2024 Jun 28;14(7):774. doi: 10.3390/biom14070774. Biomolecules. 2024. PMID: 39062488 Free PMC article.
-
Distinctive contribution of two additional residues in protein aggregation of Aβ42 and Aβ40 isoforms.BMB Rep. 2024 Jun;57(6):263-272. doi: 10.5483/BMBRep.2024-0044. BMB Rep. 2024. PMID: 38835114 Free PMC article. Review.
-
Amyloid-β1-43 cerebrospinal fluid levels and the interpretation of APP, PSEN1 and PSEN2 mutations.Alzheimers Res Ther. 2020 Sep 11;12(1):108. doi: 10.1186/s13195-020-00676-5. Alzheimers Res Ther. 2020. PMID: 32917274 Free PMC article.
-
Different Aβ43 deposition patterns in the brains of aged dogs, sea lions, and cats.J Vet Med Sci. 2022 Nov 18;84(12):1563-1573. doi: 10.1292/jvms.22-0386. Epub 2022 Oct 25. J Vet Med Sci. 2022. PMID: 36288928 Free PMC article.
-
A computational model of Alzheimer's disease at the nano, micro, and macroscales.Front Neuroinform. 2024 Mar 22;18:1348113. doi: 10.3389/fninf.2024.1348113. eCollection 2024. Front Neuroinform. 2024. PMID: 38586183 Free PMC article.
References
-
- Takami M, Nagashima Y, Sano Y, Ishihara S, Morishima-Kawashima M, Funamoto S, Ihara Y. gamma-Secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci. 2009;29(41):13042–13052. doi: 10.1523/JNEUROSCI.2362-09.2009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

