Ionizable Lipid Nanoparticle-Mediated mRNA Delivery for Human CAR T Cell Engineering
- PMID: 31951421
- PMCID: PMC7313236
- DOI: 10.1021/acs.nanolett.9b04246
Ionizable Lipid Nanoparticle-Mediated mRNA Delivery for Human CAR T Cell Engineering
Abstract
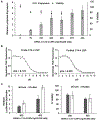
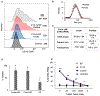
Chimeric antigen receptor (CAR) T cell therapy relies on the ex vivo manipulation of patient T cells to create potent, cancer-targeting therapies, shown to be capable of inducing remission in patients with acute lymphoblastic leukemia and large B cell lymphoma. However, current CAR T cell engineering methods use viral delivery vectors, which induce permanent CAR expression and could lead to severe adverse effects. Messenger RNA (mRNA) has been explored as a promising strategy for inducing transient CAR expression in T cells to mitigate the adverse effects associated with viral vectors, but it most commonly requires electroporation for T cell mRNA delivery, which can be cytotoxic. Here, ionizable lipid nanoparticles (LNPs) were designed for ex vivo mRNA delivery to human T cells. A library of 24 ionizable lipids was synthesized, formulated into LNPs, and screened for luciferase mRNA delivery to Jurkat cells, revealing seven formulations capable of enhanced mRNA delivery over lipofectamine. The top-performing LNP formulation, C14-4, was selected for CAR mRNA delivery to primary human T cells. This platform induced CAR expression at levels equivalent to electroporation, with substantially reduced cytotoxicity. CAR T cells engineered via C14-4 LNP treatment were then compared to electroporated CAR T cells in a coculture assay with Nalm-6 acute lymphoblastic leukemia cells, and both CAR T cell engineering methods elicited potent cancer-killing activity. These results demonstrate the ability of LNPs to deliver mRNA to primary human T cells to induce functional protein expression, and indicate the potential of LNPs to enhance mRNA-based CAR T cell engineering methods.
Keywords: CAR T; Lipid nanoparticles; T cell engineering; mRNA delivery.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Orthogonal Design of Experiments for Optimization of Lipid Nanoparticles for mRNA Engineering of CAR T Cells.Nano Lett. 2022 Jan 12;22(1):533-542. doi: 10.1021/acs.nanolett.1c02503. Epub 2021 Oct 20. Nano Lett. 2022. PMID: 34669421 Free PMC article.
-
Delivery of Plasmid DNA by Ionizable Lipid Nanoparticles to Induce CAR Expression in T Cells.Int J Nanomedicine. 2023 Oct 18;18:5891-5904. doi: 10.2147/IJN.S424723. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37873551 Free PMC article.
-
In Vivo mRNA CAR T Cell Engineering via Targeted Ionizable Lipid Nanoparticles with Extrahepatic Tropism.Small. 2024 Mar;20(11):e2304378. doi: 10.1002/smll.202304378. Epub 2023 Dec 10. Small. 2024. PMID: 38072809
-
Lipid nanoparticle (LNP) mediated mRNA delivery in cardiovascular diseases: Advances in genome editing and CAR T cell therapy.J Control Release. 2024 Aug;372:113-140. doi: 10.1016/j.jconrel.2024.06.023. Epub 2024 Jun 15. J Control Release. 2024. PMID: 38876358 Review.
-
Synergistic integration of mRNA-LNP with CAR-engineered immune cells: Pioneering progress in immunotherapy.Mol Ther. 2024 Nov 6;32(11):3772-3792. doi: 10.1016/j.ymthe.2024.09.019. Epub 2024 Sep 17. Mol Ther. 2024. PMID: 39295145 Review.
Cited by
-
Lipid nanoparticle technology for therapeutic gene regulation in the liver.Adv Drug Deliv Rev. 2020;159:344-363. doi: 10.1016/j.addr.2020.06.026. Epub 2020 Jul 2. Adv Drug Deliv Rev. 2020. PMID: 32622021 Free PMC article. Review.
-
mRNA therapeutics: New vaccination and beyond.Fundam Res. 2023 Mar 16;3(5):749-759. doi: 10.1016/j.fmre.2023.02.022. eCollection 2023 Sep. Fundam Res. 2023. PMID: 38933291 Free PMC article. Review.
-
Combinatorial development of nebulized mRNA delivery formulations for the lungs.Nat Nanotechnol. 2024 Mar;19(3):364-375. doi: 10.1038/s41565-023-01548-3. Epub 2023 Nov 20. Nat Nanotechnol. 2024. PMID: 37985700 Free PMC article.
-
Delivery of mRNA for regulating functions of immune cells.J Control Release. 2022 May;345:494-511. doi: 10.1016/j.jconrel.2022.03.033. Epub 2022 Mar 23. J Control Release. 2022. PMID: 35337940 Free PMC article. Review.
-
Development of mRNA Vaccines/Therapeutics and Their Delivery System.Mol Cells. 2023 Jan 31;46(1):41-47. doi: 10.14348/molcells.2023.2165. Epub 2022 Jan 19. Mol Cells. 2023. PMID: 36697236 Free PMC article. Review.
References
-
- Liu Y; Chen X; Han W; Zhang Y Tisagenlecleucel, an Approved Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy for the Treatment of Leukemia. Drugs Today 2017, 53 (11), 597–608. - PubMed
-
- Bouchkouj N; Kasamon YL; Claro RA de; George B; Lin X; Lee S; Blumenthal GM; Bryan W; McKee AE; Pazdur R FDA Approval Summary: Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma. Clin. Cancer Res 2019, 25 (6), 1702–1708. - PubMed
-
- Yip A; Webster RM The Market for Chimeric Antigen Receptor T Cell Therapies. Nat. Rev. Drug Discovery 2018, 17, 161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

