Hemmule: A Novel Structure with the Properties of the Stem Cell Niche
- PMID: 31947705
- PMCID: PMC7013657
- DOI: 10.3390/ijms21020539
Hemmule: A Novel Structure with the Properties of the Stem Cell Niche
Abstract
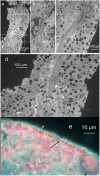
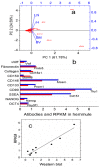
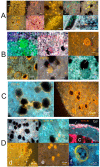
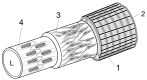
Stem cells are nurtured and regulated by a specialized microenvironment known as stem cell niche. While the functions of the niches are well defined, their structure and location remain unclear. We have identified, in rat bone marrow, the seat of hematopoietic stem cells-extensively vascularized node-like compartments that fit the requirements for stem cell niche and that we called hemmules. Hemmules are round or oval structures of about one millimeter in diameter that are surrounded by a fine capsule, have afferent and efferent vessels, are filled with the extracellular matrix and mesenchymal, hematopoietic, endothelial stem cells, and contain cells of the megakaryocyte family, which are known for homeostatic quiescence and contribution to the bone marrow environment. We propose that hemmules are the long sought hematopoietic stem cell niches and that they are prototypical of stem cell niches in other organs.
Keywords: bone marrow; endothelial; hematopoietic; megakaryocyte; mesenchymal; node; quiescence; vascular.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Influences of vascular niches on hematopoietic stem cell fate.Int J Hematol. 2014 Jun;99(6):699-705. doi: 10.1007/s12185-014-1580-4. Epub 2014 Apr 23. Int J Hematol. 2014. PMID: 24756874 Review.
-
Stem cell niche-specific Ebf3 maintains the bone marrow cavity.Genes Dev. 2018 Mar 1;32(5-6):359-372. doi: 10.1101/gad.311068.117. Epub 2018 Mar 21. Genes Dev. 2018. PMID: 29563184 Free PMC article.
-
[Bone and Stem Cells. Bone marrow microenvironment niches for hematopoietic stem and progenitor cells].Clin Calcium. 2014 Apr;24(4):517-26. Clin Calcium. 2014. PMID: 24681497 Review. Japanese.
-
Niches that regulate stem cells and hematopoiesis in adult bone marrow.Dev Cell. 2021 Jul 12;56(13):1848-1860. doi: 10.1016/j.devcel.2021.05.018. Epub 2021 Jun 18. Dev Cell. 2021. PMID: 34146467 Free PMC article. Review.
-
Adipogenic Mesenchymal Stromal Cells from Bone Marrow and Their Hematopoietic Supportive Role: Towards Understanding the Permissive Marrow Microenvironment in Acute Myeloid Leukemia.Stem Cell Rev Rep. 2016 Apr;12(2):235-44. doi: 10.1007/s12015-015-9639-z. Stem Cell Rev Rep. 2016. PMID: 26649729
Cited by
-
Analysis of key pathways and genes in nodal structure on rat skin surface using gene ontology and KEGG pathway.Genes Genomics. 2025 Jan;47(1):71-85. doi: 10.1007/s13258-024-01582-y. Epub 2024 Nov 6. Genes Genomics. 2025. PMID: 39503930
-
Bundle structures inside the deep cervical lymphatic vessels of mice.Sci Rep. 2024 Nov 18;14(1):28449. doi: 10.1038/s41598-024-80155-1. Sci Rep. 2024. PMID: 39558080 Free PMC article.
References
-
- Hooper A.T., Butler J.M., Nolan D.J., Kranz A., Iida K., Kobayashi M., Kopp H.G., Shido K., Petit I., Yanger K., et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

