Epithelial splicing regulatory protein 2-mediated alternative splicing reprograms hepatocytes in severe alcoholic hepatitis
- PMID: 31945016
- PMCID: PMC7108908
- DOI: 10.1172/JCI132691
Epithelial splicing regulatory protein 2-mediated alternative splicing reprograms hepatocytes in severe alcoholic hepatitis
Abstract
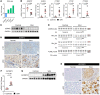
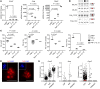
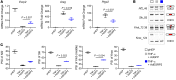
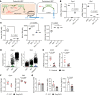
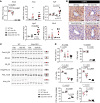
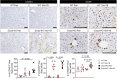
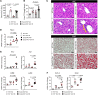
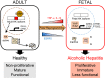
Severe alcoholic hepatitis (SAH) is a deadly liver disease without an effective medical therapy. Although SAH mortality is known to correlate with hepatic accumulation of immature liver cells, why this occurs and how it causes death are unclear. Here, we demonstrate that expression of epithelial splicing regulatory protein 2 (ESRP2), an RNA-splicing factor that maintains the nonproliferative, mature phenotype of adult hepatocytes, was suppressed in both human SAH and various mouse models of SAH in parallel with the severity of alcohol consumption and liver damage. Inflammatory cytokines released by excessive alcohol ingestion reprogrammed adult hepatocytes into proliferative, fetal-like cells by suppressing ESRP2. Sustained loss of ESRP2 permitted reemergence of a fetal RNA-splicing program that attenuates the Hippo signaling pathway and thus allows fetal transcriptional regulators to accumulate in adult liver. We further showed that depleting ESRP2 in mice exacerbated alcohol-induced steatohepatitis, enabling surviving hepatocytes to shed adult hepatocyte functions and become more regenerative, but threatening overall survival by populating the liver with functionally immature hepatocytes. Our findings revealed a mechanism that explains why liver failure develops in patients with the clinical syndrome of SAH, suggesting that recovery from SAH might be improved by limiting adult-to-fetal reprogramming in hepatocytes.
Keywords: Cell Biology; Cytokines; Hepatology; Molecular pathology; RNA processing.
Conflict of interest statement
Figures


Similar articles
-
ESRP2 controls an adult splicing programme in hepatocytes to support postnatal liver maturation.Nat Commun. 2015 Nov 4;6:8768. doi: 10.1038/ncomms9768. Nat Commun. 2015. PMID: 26531099 Free PMC article.
-
Alternative splicing rewires Hippo signaling pathway in hepatocytes to promote liver regeneration.Nat Struct Mol Biol. 2018 Oct;25(10):928-939. doi: 10.1038/s41594-018-0129-2. Epub 2018 Sep 24. Nat Struct Mol Biol. 2018. PMID: 30250226 Free PMC article.
-
Loss of ESRP2 Activates TAK1-MAPK Signaling through the Fetal RNA-Splicing Program to Promote Hepatocellular Carcinoma Progression.Adv Sci (Weinh). 2024 Jan;11(1):e2305653. doi: 10.1002/advs.202305653. Epub 2023 Nov 20. Adv Sci (Weinh). 2024. PMID: 37985644 Free PMC article.
-
New paradigms in management of alcoholic hepatitis: a review.Hepatol Int. 2017 May;11(3):255-267. doi: 10.1007/s12072-017-9790-5. Epub 2017 Feb 28. Hepatol Int. 2017. PMID: 28247264 Review.
-
MicroRNAs in alcoholic liver disease: Recent advances and future applications.J Cell Physiol. 2018 Jan;234(1):382-394. doi: 10.1002/jcp.26938. Epub 2018 Aug 4. J Cell Physiol. 2018. PMID: 30076710 Review.
Cited by
-
Clinical, histological and molecular profiling of different stages of alcohol-related liver disease.Gut. 2022 Sep;71(9):1856-1866. doi: 10.1136/gutjnl-2021-324295. Epub 2022 Jan 6. Gut. 2022. PMID: 34992134 Free PMC article.
-
Current Management and Future Treatment of Alcoholic Hepatitis.Gastroenterol Hepatol (N Y). 2020 Apr;16(4):178-189. Gastroenterol Hepatol (N Y). 2020. PMID: 34035720 Free PMC article.
-
Recent Advances in Understanding of Pathogenesis of Alcohol-Associated Liver Disease.Annu Rev Pathol. 2023 Jan 24;18:411-438. doi: 10.1146/annurev-pathmechdis-031521-030435. Epub 2022 Oct 21. Annu Rev Pathol. 2023. PMID: 36270295 Free PMC article. Review.
-
Hepatic Protein and Phosphoprotein Signatures of Alcohol-Associated Cirrhosis and Hepatitis.Am J Pathol. 2022 Jul;192(7):1066-1082. doi: 10.1016/j.ajpath.2022.04.004. Epub 2022 Apr 28. Am J Pathol. 2022. PMID: 35490715 Free PMC article.
-
Lipin 1 modulates mRNA splicing during fasting adaptation in liver.JCI Insight. 2021 Sep 8;6(17):e150114. doi: 10.1172/jci.insight.150114. JCI Insight. 2021. PMID: 34494556 Free PMC article.

