Metastable HIV-1 Surface Protein Env Sensitizes Cell Membranes to Transformation and Poration by Dual-Acting Virucidal Entry Inhibitors
- PMID: 31942789
- PMCID: PMC7362902
- DOI: 10.1021/acs.biochem.9b01008
Metastable HIV-1 Surface Protein Env Sensitizes Cell Membranes to Transformation and Poration by Dual-Acting Virucidal Entry Inhibitors
Abstract
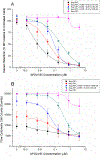
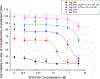
Dual-acting virucidal entry inhibitors (DAVEIs) have previously been shown to cause irreversible inactivation of HIV-1 Env-presenting pseudovirus by lytic membrane transformation. This study examined whether this transformation could be generalized to include membranes of Env-presenting cells. Flow cytometry was used to analyze HEK293T cells transiently transfected with increasing amounts of DNA encoding JRFL Env, loaded with calcein dye, and treated with serial dilutions of microvirin (Q831K/M83R)-DAVEI. Comparing calcein retention against intact Env expression (via Ab 35O22) on individual cells revealed effects proportional to Env expression. "Low-Env" cells experienced transient poration and calcein leakage, while "high-Env" cells were killed. The cell-killing effect was confirmed with an independent mitochondrial activity-based cell viability assay, showing dose-dependent cytotoxicity in response to DAVEI treatment. Transfection with increasing quantities of Env DNA showed further shifts toward "High-Env" expression and cytotoxicity, further reinforcing the Env dependence of the observed effect. Controls with unlinked DAVEI components showed no effect on calcein leakage or cell viability, confirming a requirement for covalently linked DAVEI compounds to achieve Env transformation. These data demonstrate that the metastability of Env is an intrinsic property of the transmembrane protein complex and can be perturbed to cause membrane disruption in both virus and cell contexts.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures



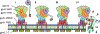
Similar articles
-
Peptide Triazole Thiol Irreversibly Inactivates Metastable HIV-1 Env by Accessing Conformational Triggers Intrinsic to Virus-Cell Entry.Microorganisms. 2021 Jun 12;9(6):1286. doi: 10.3390/microorganisms9061286. Microorganisms. 2021. PMID: 34204725 Free PMC article.
-
Restricted HIV-1 Env glycan engagement by lectin-reengineered DAVEI protein chimera is sufficient for lytic inactivation of the virus.Biochem J. 2018 Mar 9;475(5):931-957. doi: 10.1042/BCJ20170662. Biochem J. 2018. PMID: 29343613 Free PMC article.
-
Lytic Inactivation of Human Immunodeficiency Virus by Dual Engagement of gp120 and gp41 Domains in the Virus Env Protein Trimer.Biochemistry. 2016 Nov 8;55(44):6100-6114. doi: 10.1021/acs.biochem.6b00570. Epub 2016 Oct 27. Biochemistry. 2016. PMID: 27731975 Free PMC article.
-
HIV envelope: challenges and opportunities for development of entry inhibitors.Trends Microbiol. 2011 Apr;19(4):191-7. doi: 10.1016/j.tim.2011.02.001. Epub 2011 Mar 4. Trends Microbiol. 2011. PMID: 21377881 Free PMC article. Review.
-
HIV-1 diversity in the envelope glycoproteins: implications for viral entry inhibition.Viruses. 2013 Feb 6;5(2):595-604. doi: 10.3390/v5020595. Viruses. 2013. PMID: 23389465 Free PMC article. Review.
Cited by
-
HIV-1 Env-Dependent Cell Killing by Bifunctional Small-Molecule/Peptide Conjugates.ACS Chem Biol. 2021 Jan 15;16(1):193-204. doi: 10.1021/acschembio.0c00888. Epub 2021 Jan 7. ACS Chem Biol. 2021. PMID: 33410670 Free PMC article.
-
Altered Env conformational dynamics as a mechanism of resistance to peptide-triazole HIV-1 inactivators.Retrovirology. 2021 Oct 9;18(1):31. doi: 10.1186/s12977-021-00575-z. Retrovirology. 2021. PMID: 34627310 Free PMC article.
-
Irreversible Inactivation of SARS-CoV-2 by Lectin Engagement with Two Glycan Clusters on the Spike Protein.Biochemistry. 2023 Jul 18;62(14):2115-2127. doi: 10.1021/acs.biochem.3c00109. Epub 2023 Jun 21. Biochemistry. 2023. PMID: 37341186 Free PMC article.
-
Peptide Triazole Thiol Irreversibly Inactivates Metastable HIV-1 Env by Accessing Conformational Triggers Intrinsic to Virus-Cell Entry.Microorganisms. 2021 Jun 12;9(6):1286. doi: 10.3390/microorganisms9061286. Microorganisms. 2021. PMID: 34204725 Free PMC article.
References
-
- Clinical Guidelines: Antiretroviral Therapy In Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach, 2nd ed. (2016) World Health Organization, Geneva. - PubMed
-
- Gravatt LAH, Leibrand CR, Patel S, and McRae M (2017) New Drugs in the Pipeline for the Treatment of HIV: a Review. Current Infectious Disease Reports 19 (11), 42. - PubMed
-
- Xu F, Acosta EP, Liang L, He Y, Yang J, Kerstner-Wood C, Zheng Q, Huang J, and Wang K (2017) Current Status of the Pharmacokinetics and Pharmacodynamics of HIV-1 Entry Inhibitors and HIV Therapy. Curr. Drug Metab 18, 769–781. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

