Generation of an immunodeficient mouse model of tcirg1-deficient autosomal recessive osteopetrosis
- PMID: 31938717
- PMCID: PMC6953598
- DOI: 10.1016/j.bonr.2020.100242
Generation of an immunodeficient mouse model of tcirg1-deficient autosomal recessive osteopetrosis
Abstract
Background: Autosomal recessive osteopetrosis is a rare skeletal disorder with increased bone density due to a failure in osteoclast bone resorption. In most cases, the defect is cell-autonomous, and >50% of patients bear mutations in the TCIRG1 gene, encoding for a subunit of the vacuolar proton pump essential for osteoclast resorptive activity. The only cure is hematopoietic stem cell transplantation, which corrects the bone pathology by allowing the formation of donor-derived functional osteoclasts. Therapeutic approaches using patient-derived cells corrected ex vivo through viral transduction or gene editing can be considered, but to date functional rescue cannot be demonstrated in vivo because a relevant animal model for xenotransplant is missing.
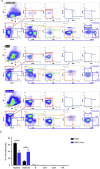
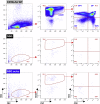
Methods: We generated a new mouse model, which we named NSG oc/oc, presenting severe autosomal recessive osteopetrosis owing to the Tcirg1 oc mutation, and profound immunodeficiency caused by the NSG background. We performed neonatal murine bone marrow transplantation and xenotransplantation with human CD34+ cells.
Results: We demonstrated that neonatal murine bone marrow transplantation rescued NSG oc/oc mice, in line with previous findings in the oc/oc parental strain and with evidence from clinical practice in humans. Importantly, we also demonstrated human cell chimerism in the bone marrow of NSG oc/oc mice transplanted with human CD34+ cells. The severity and rapid progression of the disease in the mouse model prevented amelioration of the bone pathology; nevertheless, we cannot completely exclude that minor early modifications of the bone tissue might have occurred.
Conclusion: Our work paves the way to generating an improved xenograft model for in vivo evaluation of functional rescue of patient-derived corrected cells. Further refinement of the newly generated mouse model will allow capitalizing on it for an optimized exploitation in the path to novel cell therapies.
Keywords: Mouse model; Osteopetrosis; Transplantation.
© 2020 Published by Elsevier Inc.
Figures



Similar articles
-
Hematopoietic Stem Cell-Targeted Neonatal Gene Therapy with a Clinically Applicable Lentiviral Vector Corrects Osteopetrosis in oc/oc Mice.Hum Gene Ther. 2019 Nov;30(11):1395-1404. doi: 10.1089/hum.2019.047. Epub 2019 Jul 3. Hum Gene Ther. 2019. PMID: 31179768
-
Fetal liver cells transplanted in utero rescue the osteopetrotic phenotype in the oc/oc mouse.Am J Pathol. 2009 Mar;174(3):727-35. doi: 10.2353/ajpath.2009.080688. Epub 2009 Feb 13. Am J Pathol. 2009. PMID: 19218349 Free PMC article.
-
Targeting NSG Mice Engrafting Cells with a Clinically Applicable Lentiviral Vector Corrects Osteoclasts in Infantile Malignant Osteopetrosis.Hum Gene Ther. 2018 Aug;29(8):938-949. doi: 10.1089/hum.2017.053. Epub 2017 Oct 3. Hum Gene Ther. 2018. PMID: 28726516
-
Recent developments in the understanding of the pathophysiology of osteopetrosis.Eur J Endocrinol. 1996 Feb;134(2):143-56. doi: 10.1530/eje.0.1340143. Eur J Endocrinol. 1996. PMID: 8630510 Review.
-
Genetics, pathogenesis and complications of osteopetrosis.Bone. 2008 Jan;42(1):19-29. doi: 10.1016/j.bone.2007.08.029. Epub 2007 Aug 30. Bone. 2008. PMID: 17936098 Review.
Cited by
-
Autosomal recessive osteopetrosis: mechanisms and treatments.Dis Model Mech. 2021 May 1;14(5):dmm048940. doi: 10.1242/dmm.048940. Epub 2021 May 10. Dis Model Mech. 2021. PMID: 33970241 Free PMC article. Review.
-
Gene therapy for infantile malignant osteopetrosis: review of pre-clinical research and proof-of-concept for phenotypic reversal.Mol Ther Methods Clin Dev. 2020 Dec 25;20:389-397. doi: 10.1016/j.omtm.2020.12.009. eCollection 2021 Mar 12. Mol Ther Methods Clin Dev. 2020. PMID: 33575431 Free PMC article. Review.
-
Rankl genetic deficiency and functional blockade undermine skeletal stem and progenitor cell differentiation.Stem Cell Res Ther. 2024 Jul 6;15(1):203. doi: 10.1186/s13287-024-03803-3. Stem Cell Res Ther. 2024. PMID: 38971808 Free PMC article.
-
Molecular Mechanisms of Craniofacial and Dental Abnormalities in Osteopetrosis.Int J Mol Sci. 2023 Jun 20;24(12):10412. doi: 10.3390/ijms241210412. Int J Mol Sci. 2023. PMID: 37373559 Free PMC article. Review.
-
Correction of osteopetrosis in the neonate oc/oc murine model after lentiviral vector gene therapy and non-genotoxic conditioning.Front Endocrinol (Lausanne). 2024 Sep 9;15:1450349. doi: 10.3389/fendo.2024.1450349. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39314524 Free PMC article.
References
-
- Abkowitz J.L., Golinelli D., Harrison D.E., Guttorp P. In vivo kinetics of murine hemopoietic stem cells. Blood. 2000;96(10):3399–3405. - PubMed
-
- Askmyr M., Flores C., Fasth A., Richter J. Prospects for gene therapy of osteopetrosis. Current Gene Therapy. 2009;9(3):150–159. - PubMed
-
- Askmyr M., Holmberg J., Flores C., Ehinger M., Hjalt T., Richter J. Low-dose busulphan conditioning and neonatal stem cell transplantation preserves vision and restores hematopoiesis in severe murine osteopetrosis. Exp. Hematol. 2009;37(2):302–308. - PubMed
-
- Bahr T.L., Lund T., Sando N.M., Orchard P.J., Miller W.P. Haploidentical transplantation with post-transplant cyclophosphamide following reduced-intensity conditioning for osteopetrosis: outcomes in three children. Bone Marrow Transplant. 2016;51(11):1546–1548. - PubMed
-
- Blin-Wakkach C., Breuil V., Quincey D., Bagnis C., Carle G.F. Establishment and characterization of new osteoclast progenitor cell lines derived from osteopetrotic and wild type mice. Bone. 2006;39(1):53–60. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

