Tracing the Dynamics of Stem Cell Fate
- PMID: 31932319
- PMCID: PMC7263083
- DOI: 10.1101/cshperspect.a036202
Tracing the Dynamics of Stem Cell Fate
Abstract
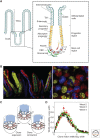
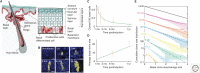
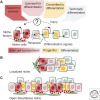
The mechanisms that regulate the balance between stem cell duplication and differentiation in adult tissues remain in debate. Using a combination of genetic lineage tracing and marker-based assays, the quantitative statistical analysis of clone size and cell composition has provided insights into the patterns of stem cell fate across a variety of tissue types and organisms. These studies have emphasized the role of niche factors and environmental cues in promoting stem cell competence, fate priming, and stochastic renewal programs. At the same time, evidence for injury-induced "cellular reprogramming" has revealed the remarkable flexibility of cell states, allowing progenitors to reacquire self-renewal potential during regeneration. Together, these findings have questioned the nature of stem cell identity and function. Here, focusing on a range of canonical tissue types, we review how quantitative modeling-based approaches have uncovered conserved patterns of stem cell fate and provided new insights into the mechanisms that regulate self-renewal.
Copyright © 2020 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


Similar articles
-
Cycling progenitors maintain epithelia while diverse cell types contribute to repair.Bioessays. 2013 May;35(5):443-51. doi: 10.1002/bies.201200166. Epub 2013 Mar 6. Bioessays. 2013. PMID: 23463676 Review.
-
Injury Activates Transient Olfactory Stem Cell States with Diverse Lineage Capacities.Cell Stem Cell. 2017 Dec 7;21(6):775-790.e9. doi: 10.1016/j.stem.2017.10.014. Epub 2017 Nov 22. Cell Stem Cell. 2017. PMID: 29174333 Free PMC article.
-
Tracing cellular dynamics in tissue development, maintenance and disease.Curr Opin Cell Biol. 2016 Dec;43:38-45. doi: 10.1016/j.ceb.2016.07.001. Epub 2016 Jul 27. Curr Opin Cell Biol. 2016. PMID: 27474807 Review.
-
Homeostatic Epidermal Stem Cell Self-Renewal Is Driven by Local Differentiation.Cell Stem Cell. 2018 Nov 1;23(5):677-686.e4. doi: 10.1016/j.stem.2018.09.005. Epub 2018 Sep 27. Cell Stem Cell. 2018. PMID: 30269903 Free PMC article.
-
Universal patterns of stem cell fate in cycling adult tissues.Development. 2011 Aug;138(15):3103-11. doi: 10.1242/dev.060103. Development. 2011. PMID: 21750026
Cited by
-
rDNA magnification is a unique feature of germline stem cells.Proc Natl Acad Sci U S A. 2023 Nov 21;120(47):e2314440120. doi: 10.1073/pnas.2314440120. Epub 2023 Nov 15. Proc Natl Acad Sci U S A. 2023. PMID: 37967216 Free PMC article.
-
Mouse Spermatogenesis Reflects the Unity and Diversity of Tissue Stem Cell Niche Systems.Cold Spring Harb Perspect Biol. 2020 Dec 1;12(12):a036186. doi: 10.1101/cshperspect.a036186. Cold Spring Harb Perspect Biol. 2020. PMID: 32152184 Free PMC article. Review.
-
Single-cell expression profile of Drosophila ovarian follicle stem cells illuminates spatial differentiation in the germarium.BMC Biol. 2023 Jun 20;21(1):143. doi: 10.1186/s12915-023-01636-9. BMC Biol. 2023. PMID: 37340484 Free PMC article.
-
Cerebral organoids display dynamic clonal growth and tunable tissue replenishment.Nat Cell Biol. 2024 May;26(5):710-718. doi: 10.1038/s41556-024-01412-z. Epub 2024 May 7. Nat Cell Biol. 2024. PMID: 38714853 Free PMC article.
-
Emergent order in epithelial sheets by interplay of cell divisions and cell fate regulation.PLoS Comput Biol. 2024 Oct 14;20(10):e1012465. doi: 10.1371/journal.pcbi.1012465. eCollection 2024 Oct. PLoS Comput Biol. 2024. PMID: 39401252 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
